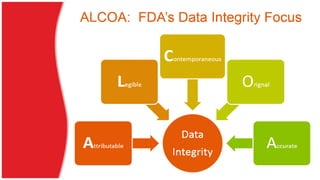
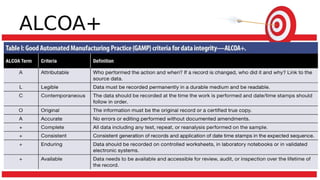
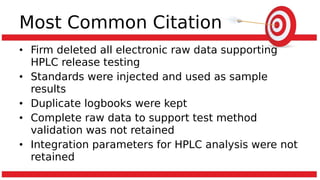
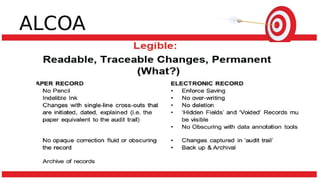
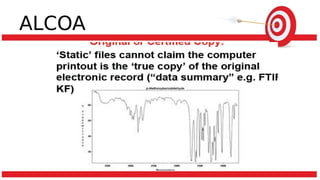
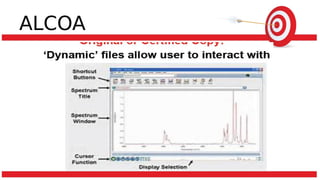
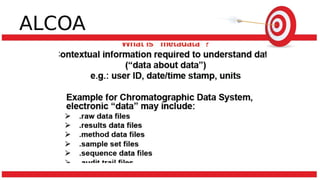
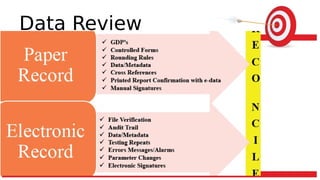
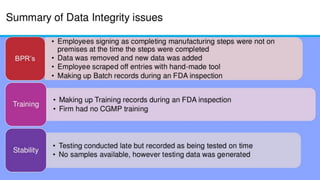
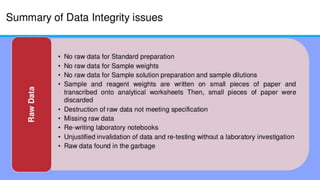
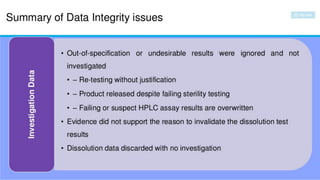
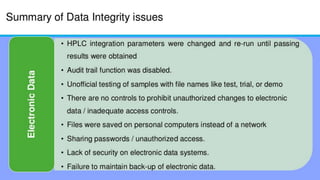
The document discusses common data integrity issues found during FDA inspections of pharmaceutical companies. It lists the most common citations, including failures to document production processes and laboratory activities at the time they occurred, manipulate or delete electronic records and data, investigate batch failures, and ensure complete and accurate documentation is maintained to support batch releases. Maintaining rigorous data integrity controls is important to ensure the reliability and trustworthiness of data and avoid regulatory issues.