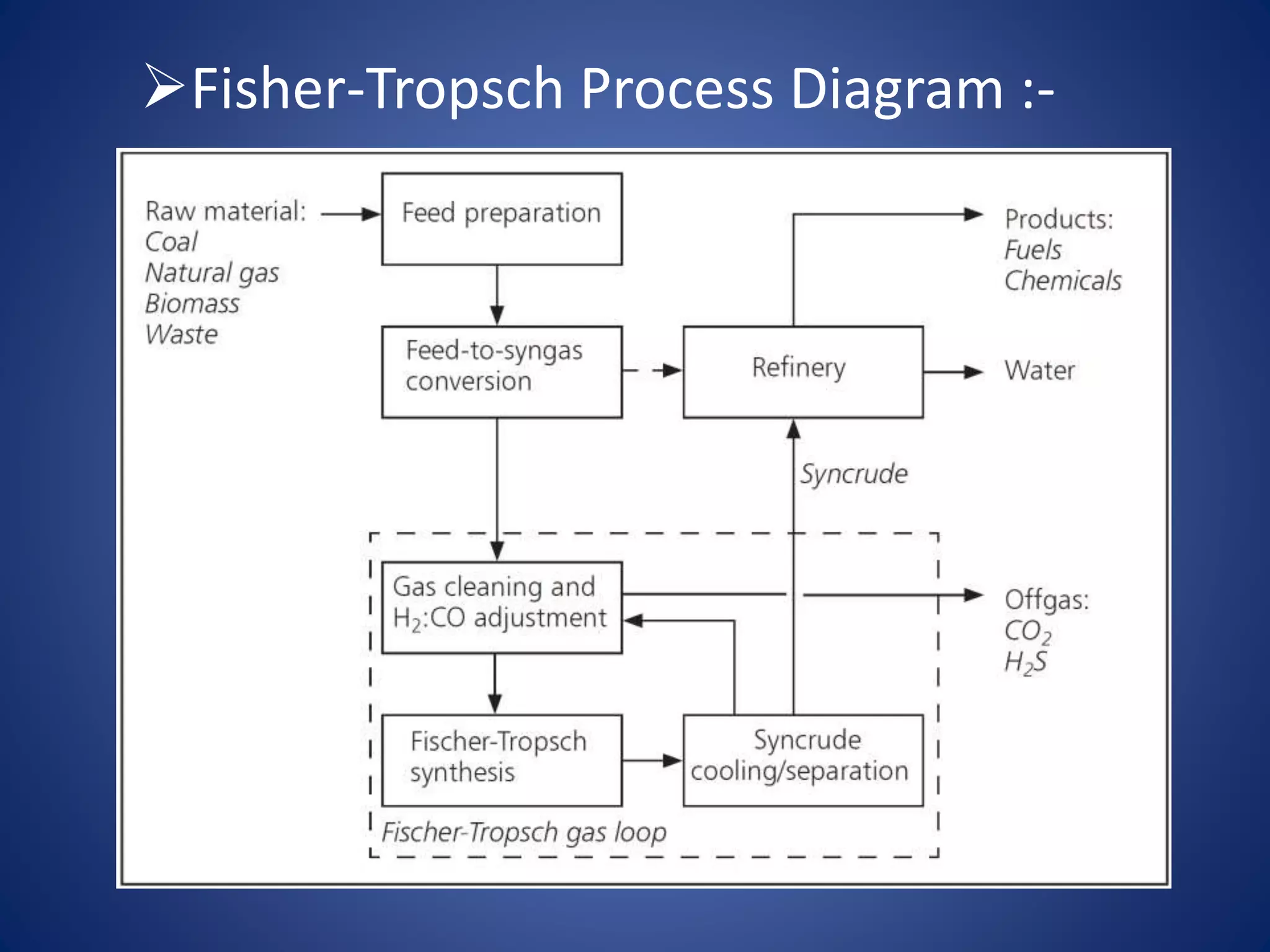
The document discusses petroleum refining, cracking, and methods of producing synthetic petrol. It describes how crude oil is refined through separation, conversion, and treatment processes like distillation. Cracking breaks large hydrocarbon molecules into smaller, more useful molecules through thermal or catalytic cracking. Synthetic petrol can be produced via polymerization, Fischer-Tropsch synthesis from syngas, or Bergius process where coal is hydrogenated over a catalyst into liquid fuels.