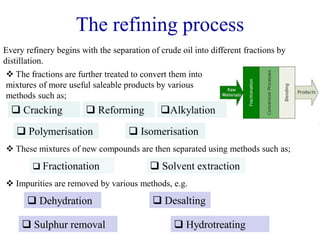
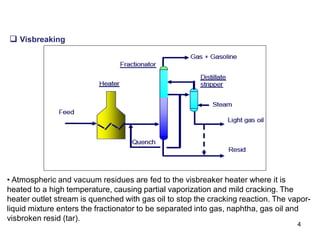
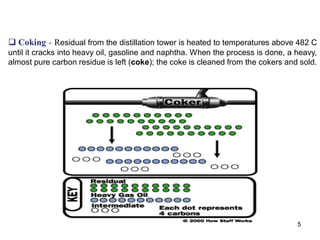
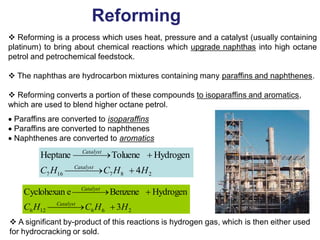
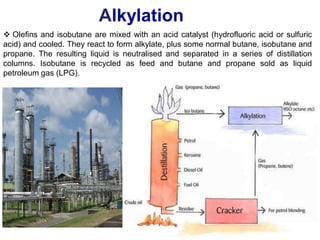
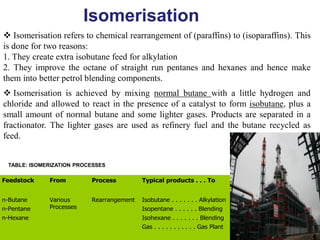
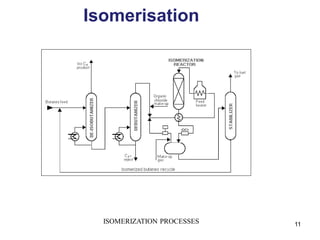
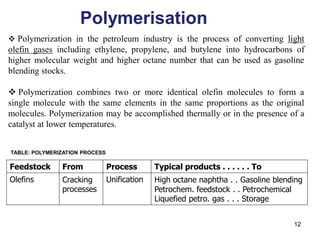
The refining process of crude oil involves separating it into different fractions through distillation, followed by various methods like cracking, reforming, isomerization, alkylation, and polymerization to produce useful products. Cracking techniques, including thermal and catalytic methods, break down larger hydrocarbons into smaller, more valuable compounds, while hydrotreating is used to remove impurities from the products. Additionally, processes like isomerization and alkylation enhance the octane rating of fuels, contributing to the efficiency and quality of the final products.