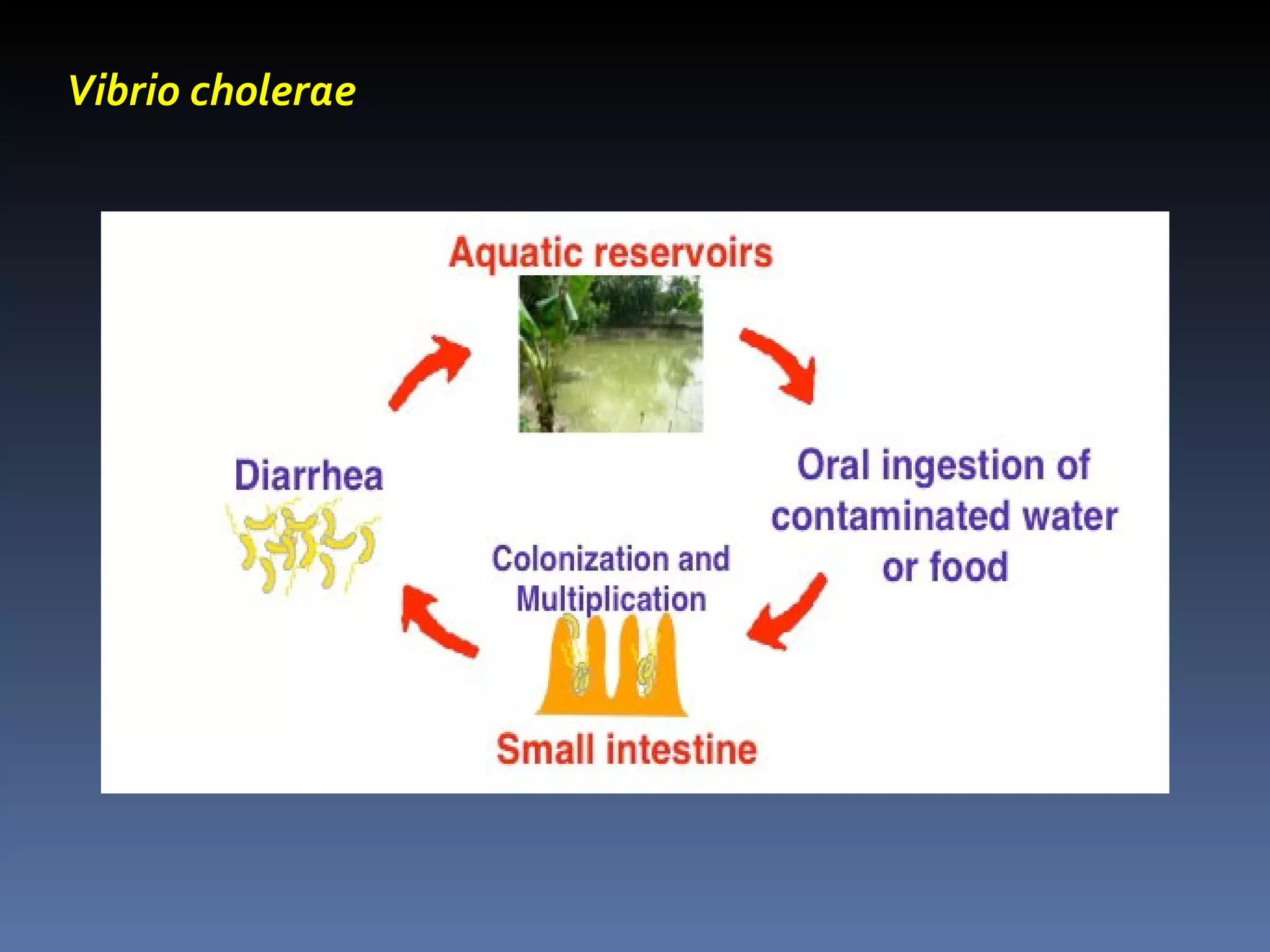
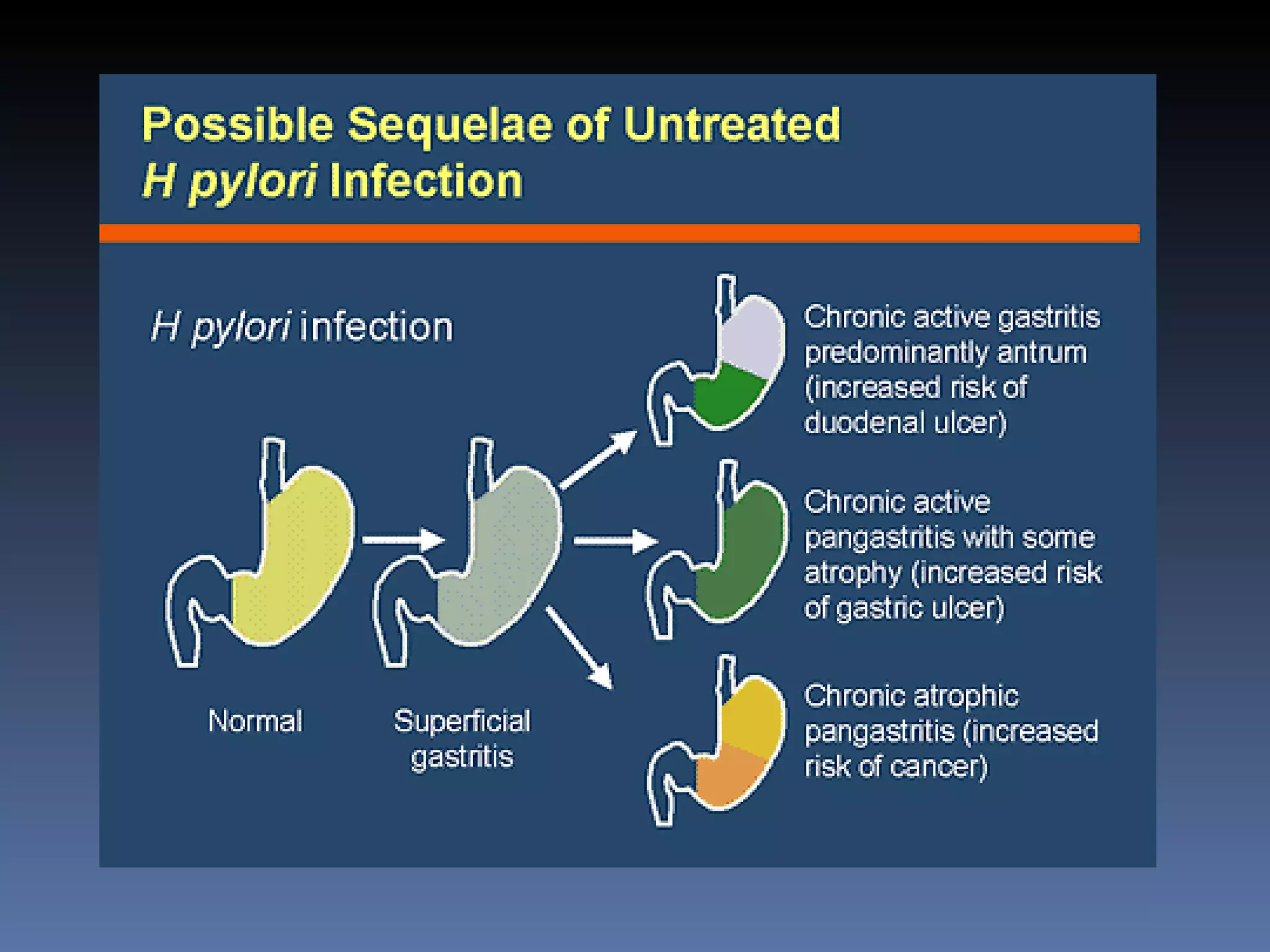
The document discusses several gram-negative bacteria including Vibrios, Pseudomonas, Campylobacter, Helicobacter, Haemophilus, Bordetella, and Brucella. It describes their characteristics, habitats, mechanisms of infection, diseases caused, methods of diagnosis and treatment. Key points covered include that Vibrio cholerae causes cholera, Pseudomonas aeruginosa causes opportunistic infections, Campylobacter jejuni is a common cause of food poisoning, and Haemophilus influenzae was a major cause of childhood meningitis prior to vaccination.