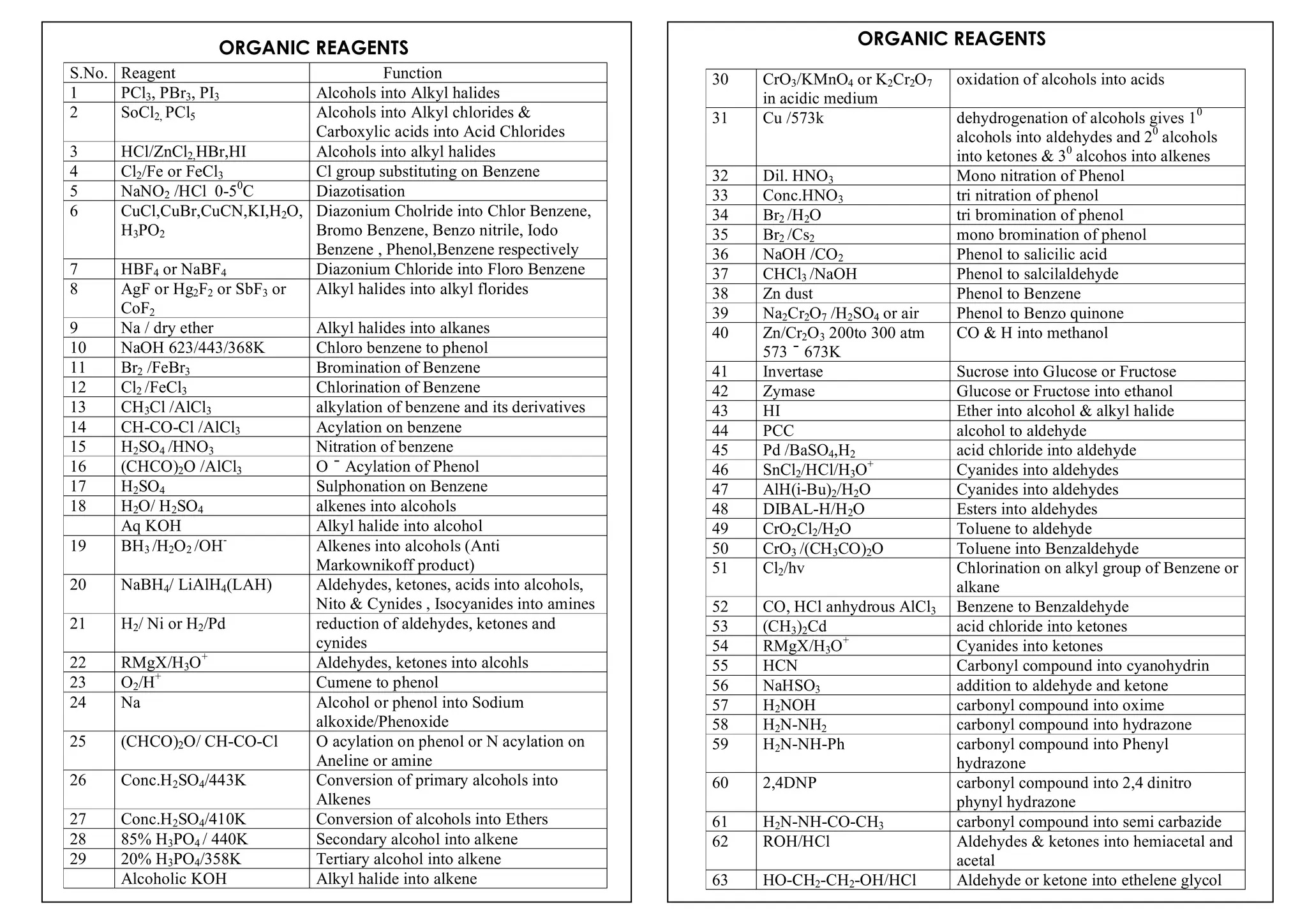
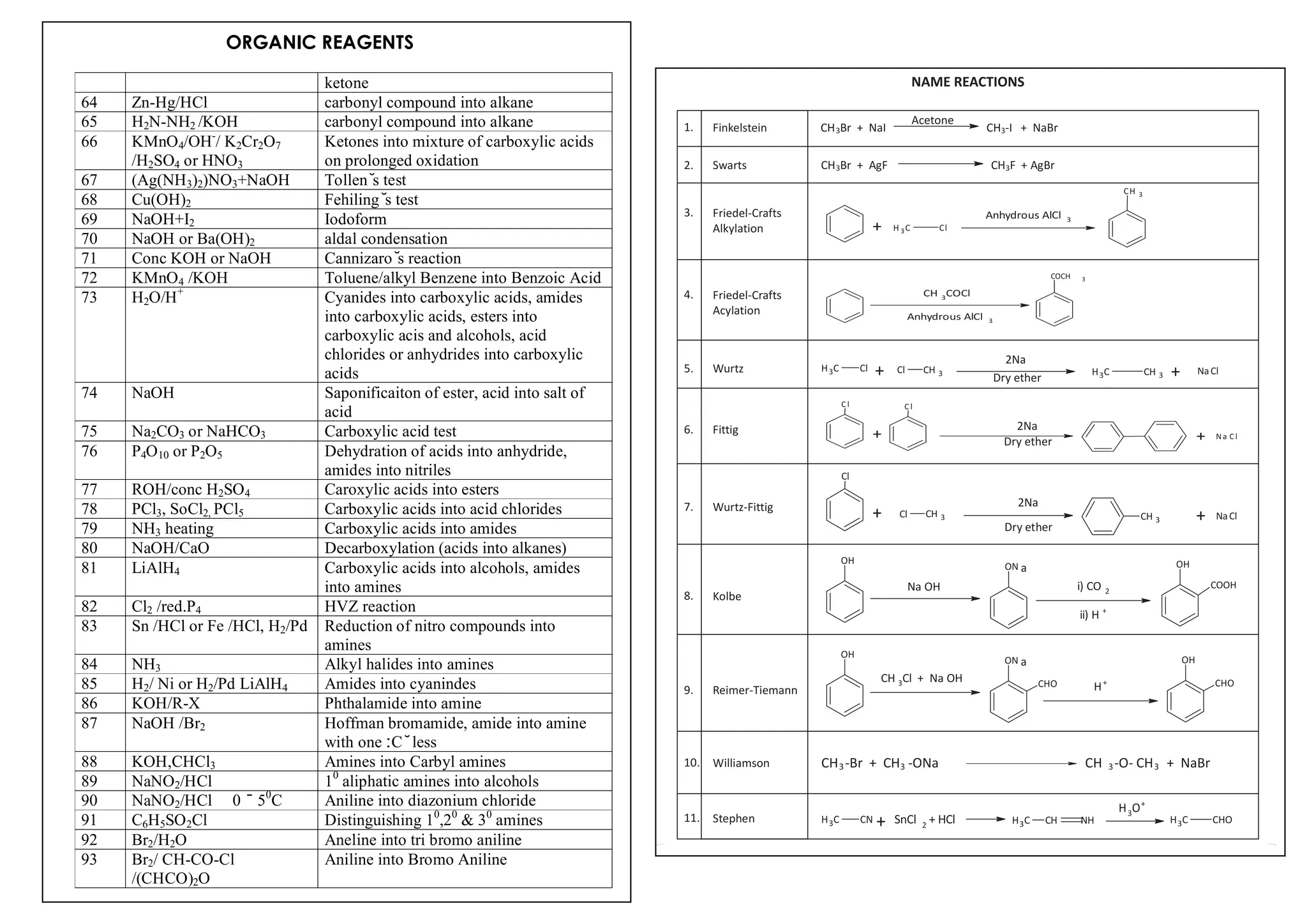
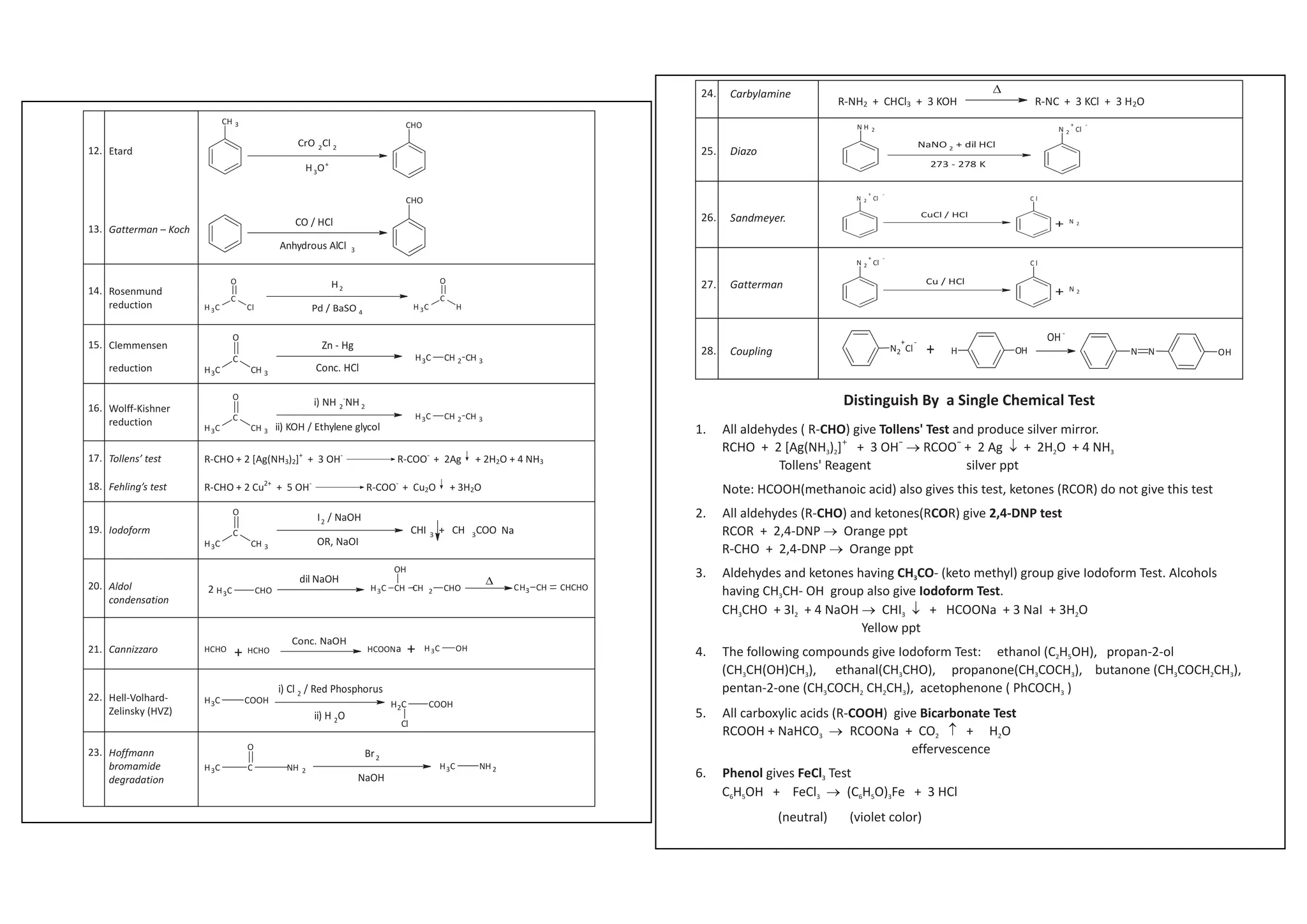
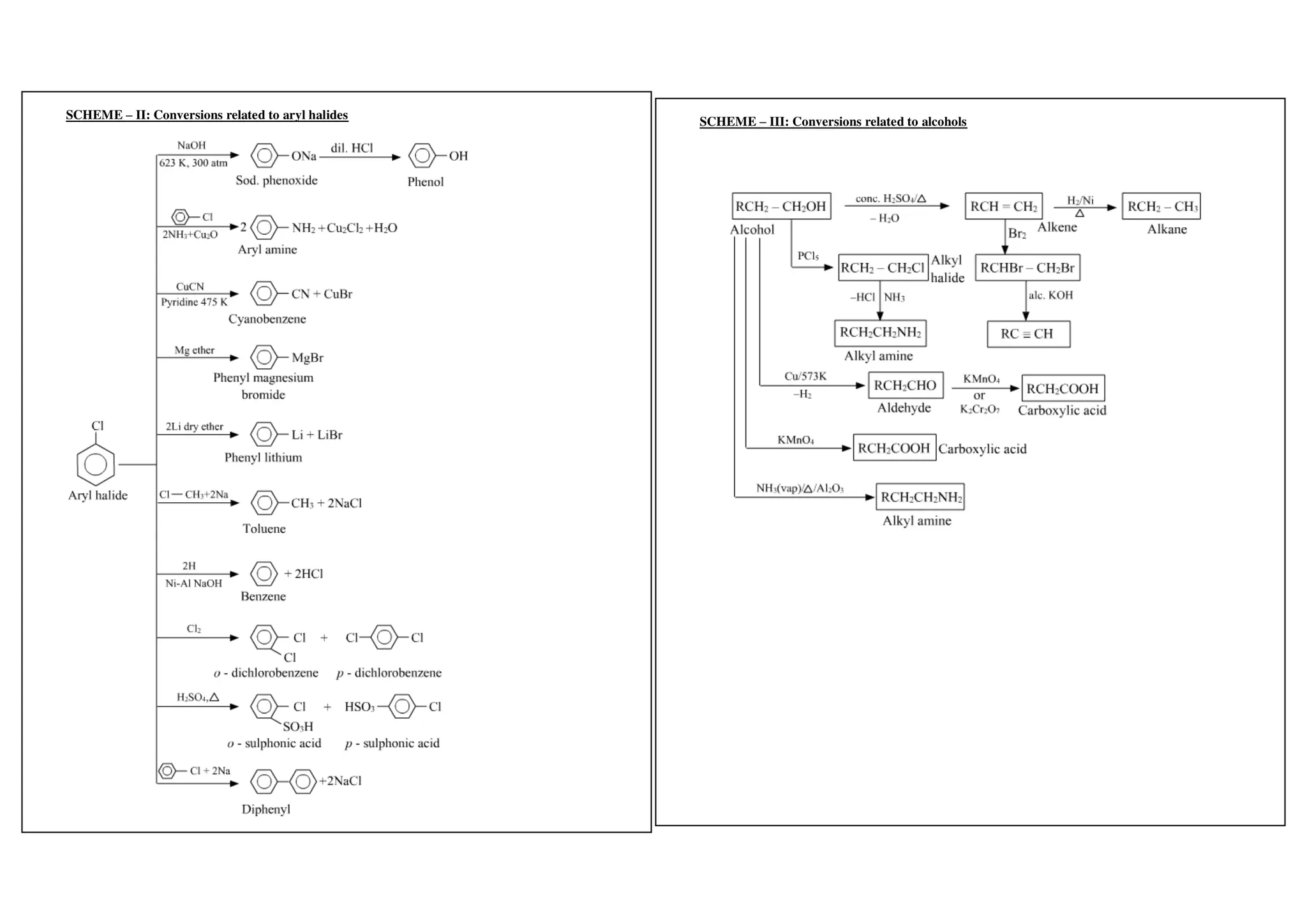
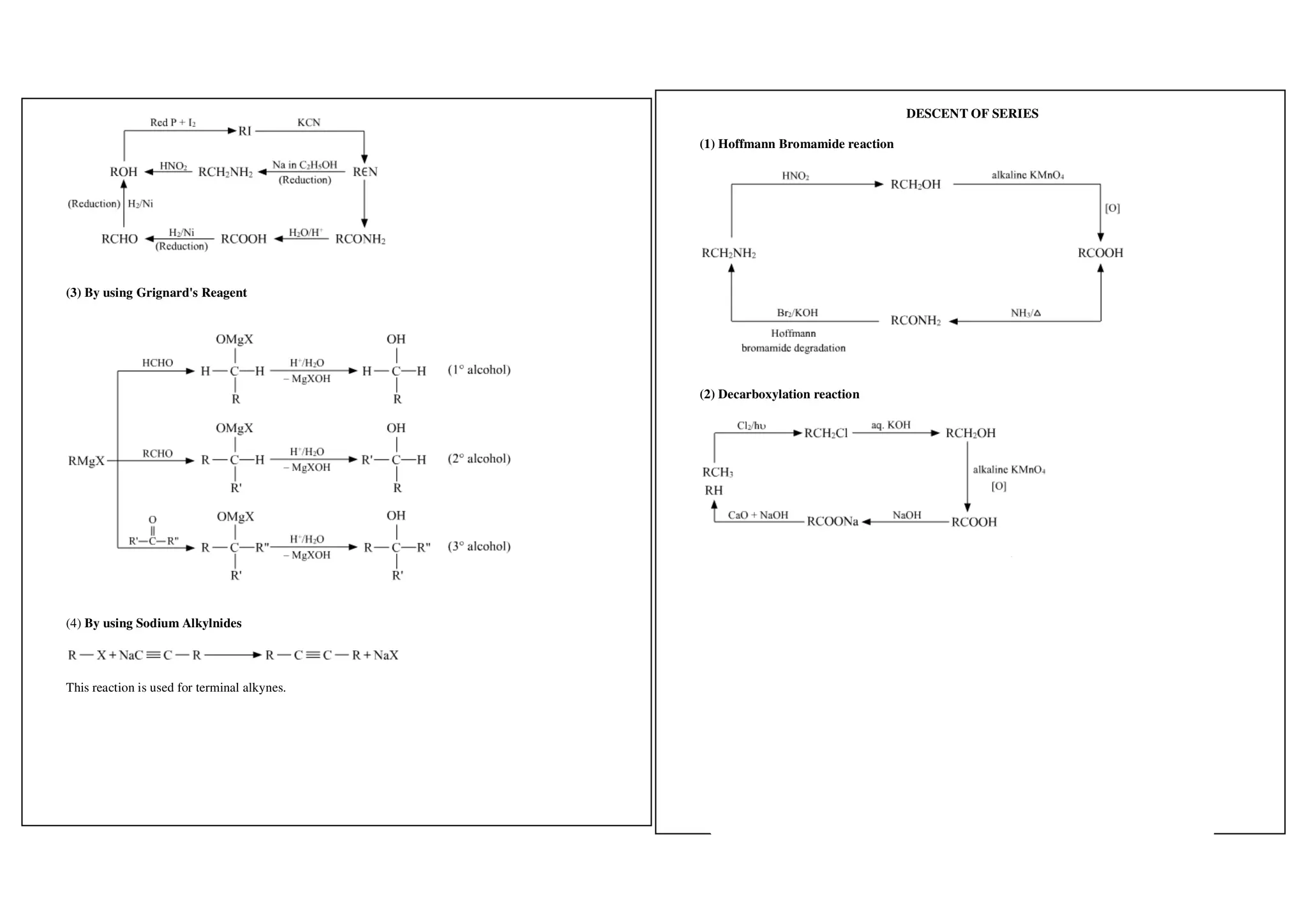
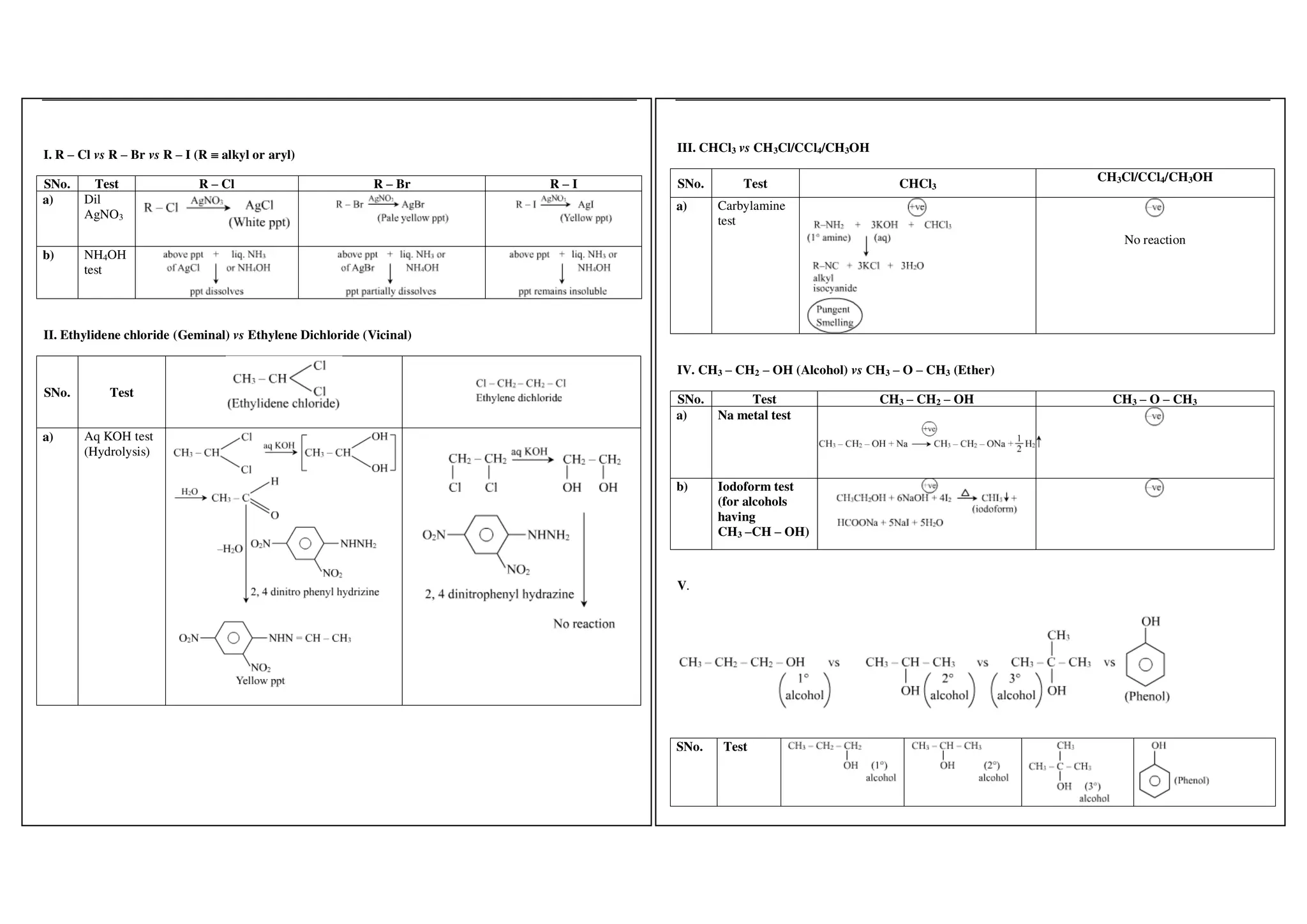
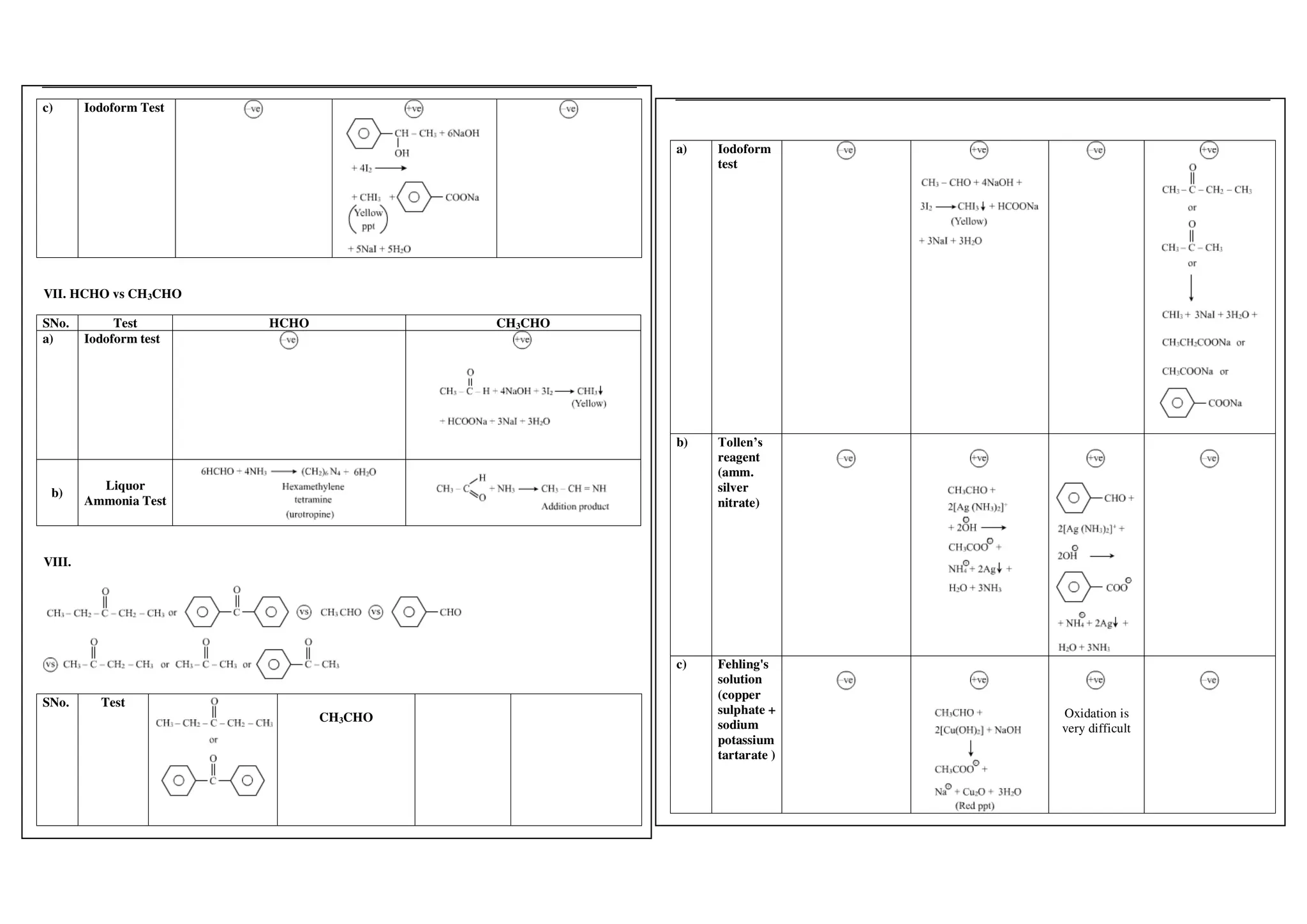
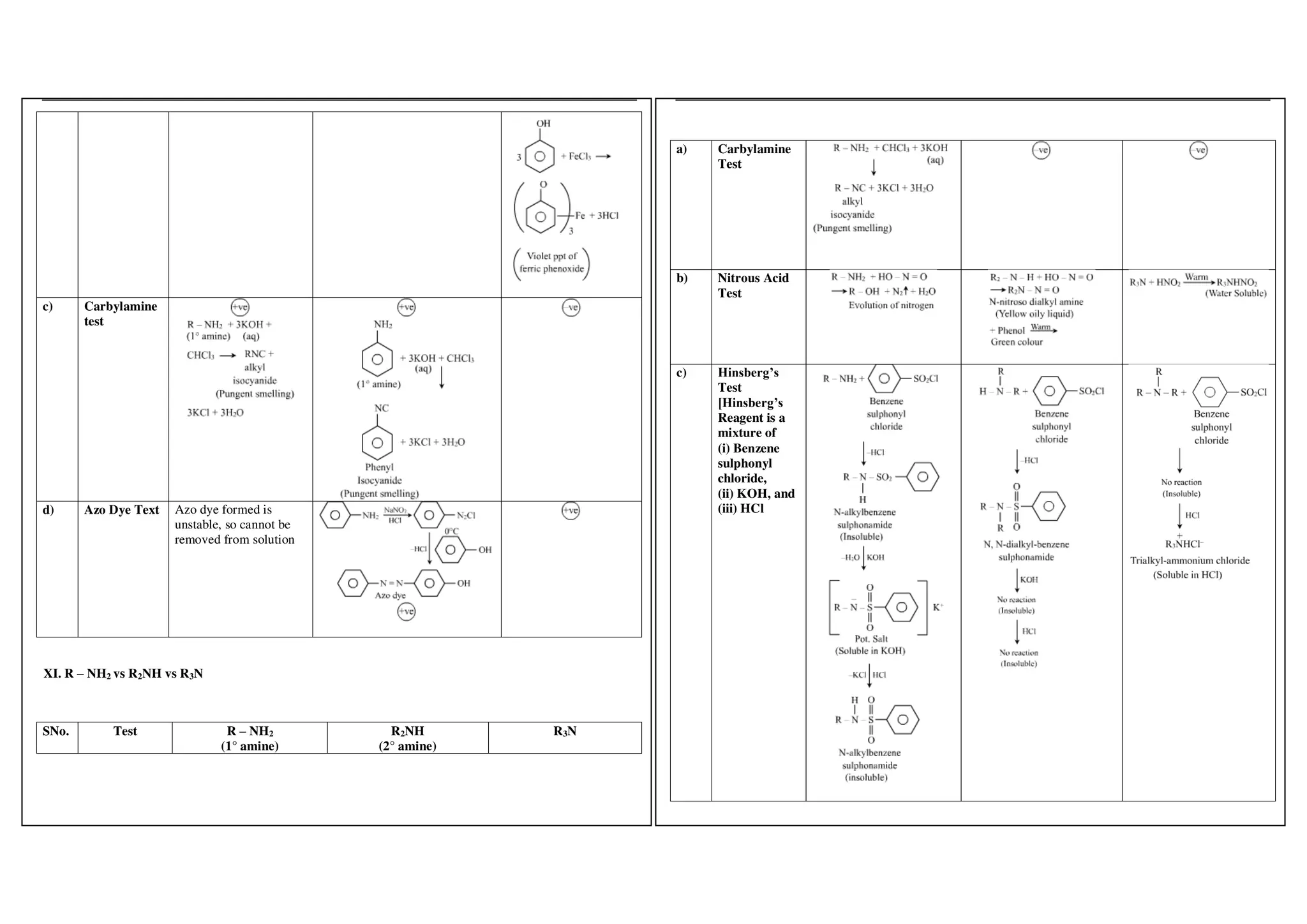
I. Conversions related to organic reagents and functional groups include reactions of alkyl halides, aryl halides, alcohols, phenols, aldehydes, ketones, carboxylic acids and derivatives, and alkyl and aryl amines.
II. Key reactions involve substitution, elimination, oxidation, reduction, hydrolysis, dehydration, decarboxylation and other functional group interconversions.
III. Reagents used include strong acids and bases, oxidizing and reducing agents, halogens, organometallic reagents, and other common organic reagents.