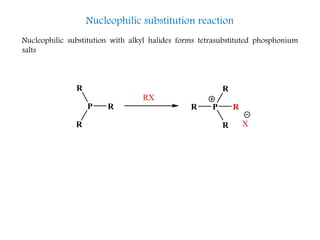
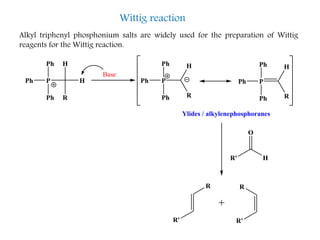
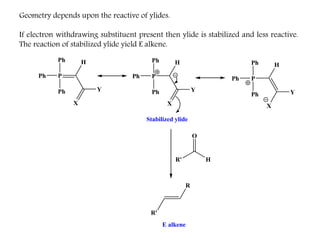
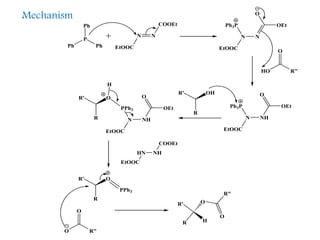
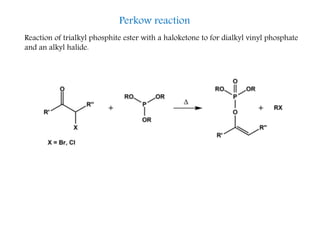
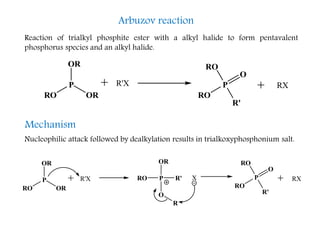
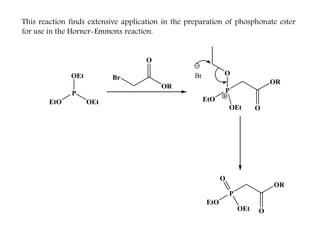
This document discusses organophosphorus compounds, which contain carbon-phosphorus bonds. It provides an overview of different classes of organophosphorus compounds such as phosphines, phosphonium salts, phosphine oxides, and their properties. It also summarizes some important reactions of phosphines including nucleophilic substitution, Staudinger reduction, and Mitsunobu reaction. Methods for synthesizing phosphines like the reaction of organometallic reagents with phosphorus halides are also briefly outlined.