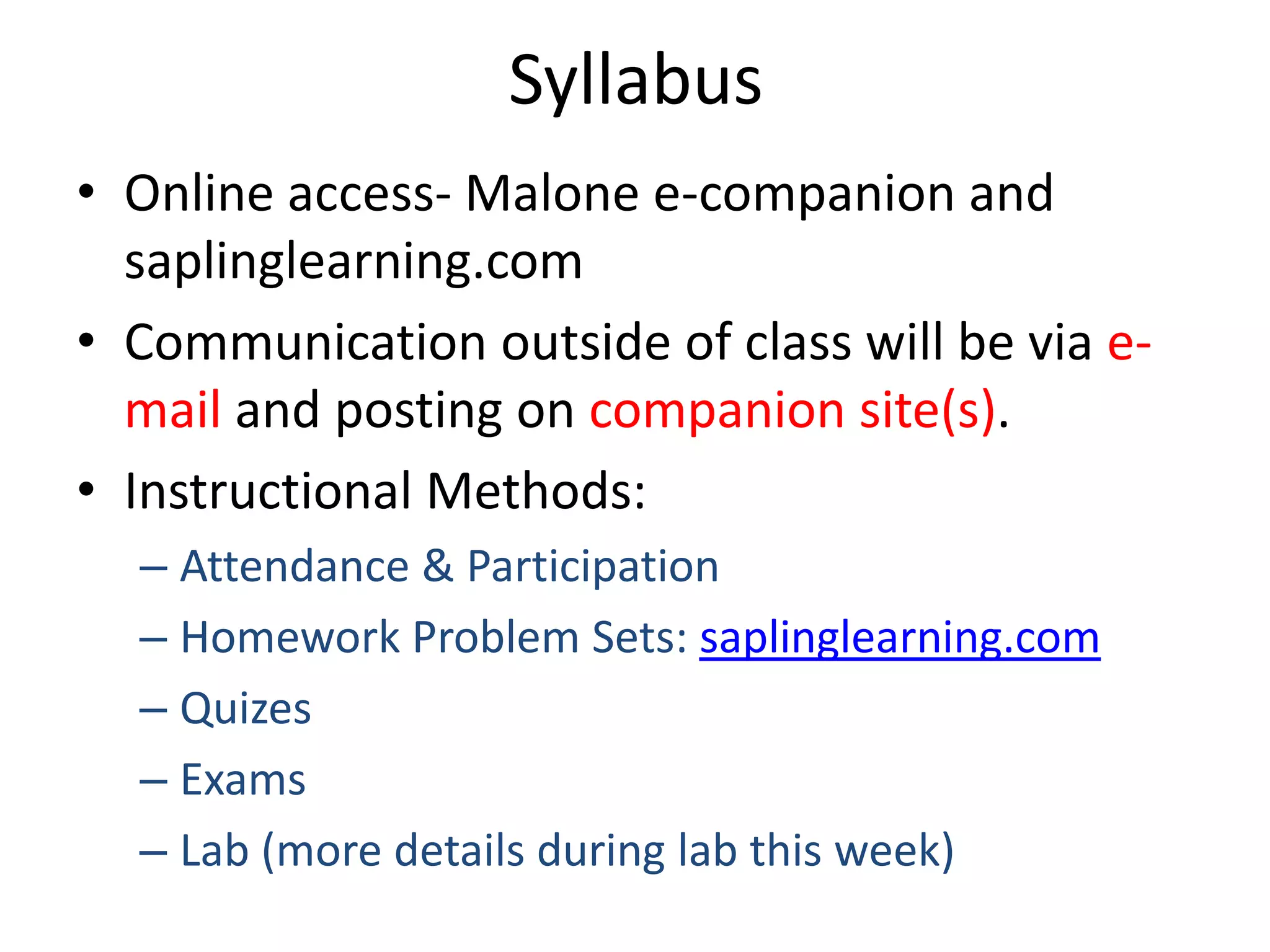
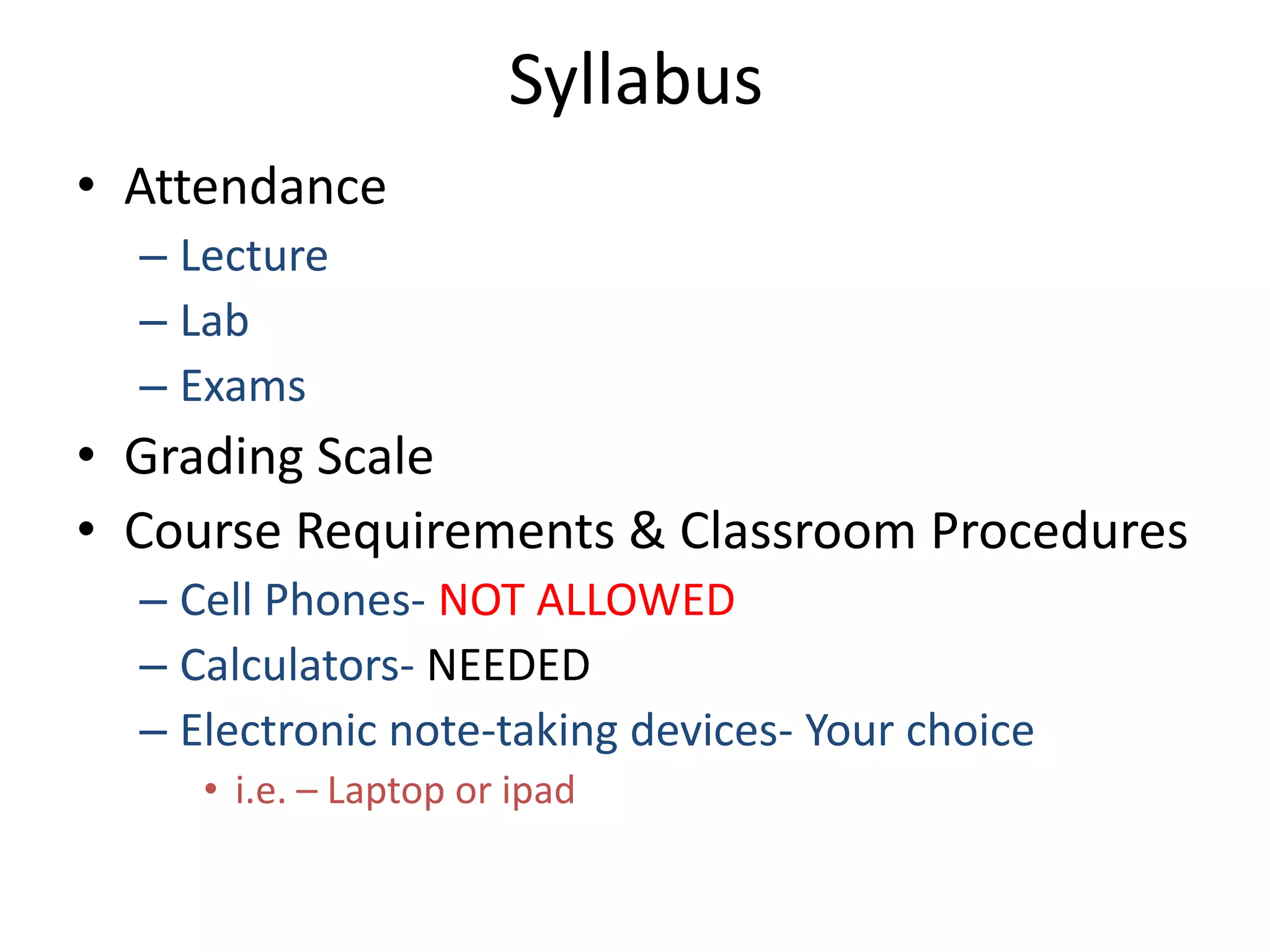
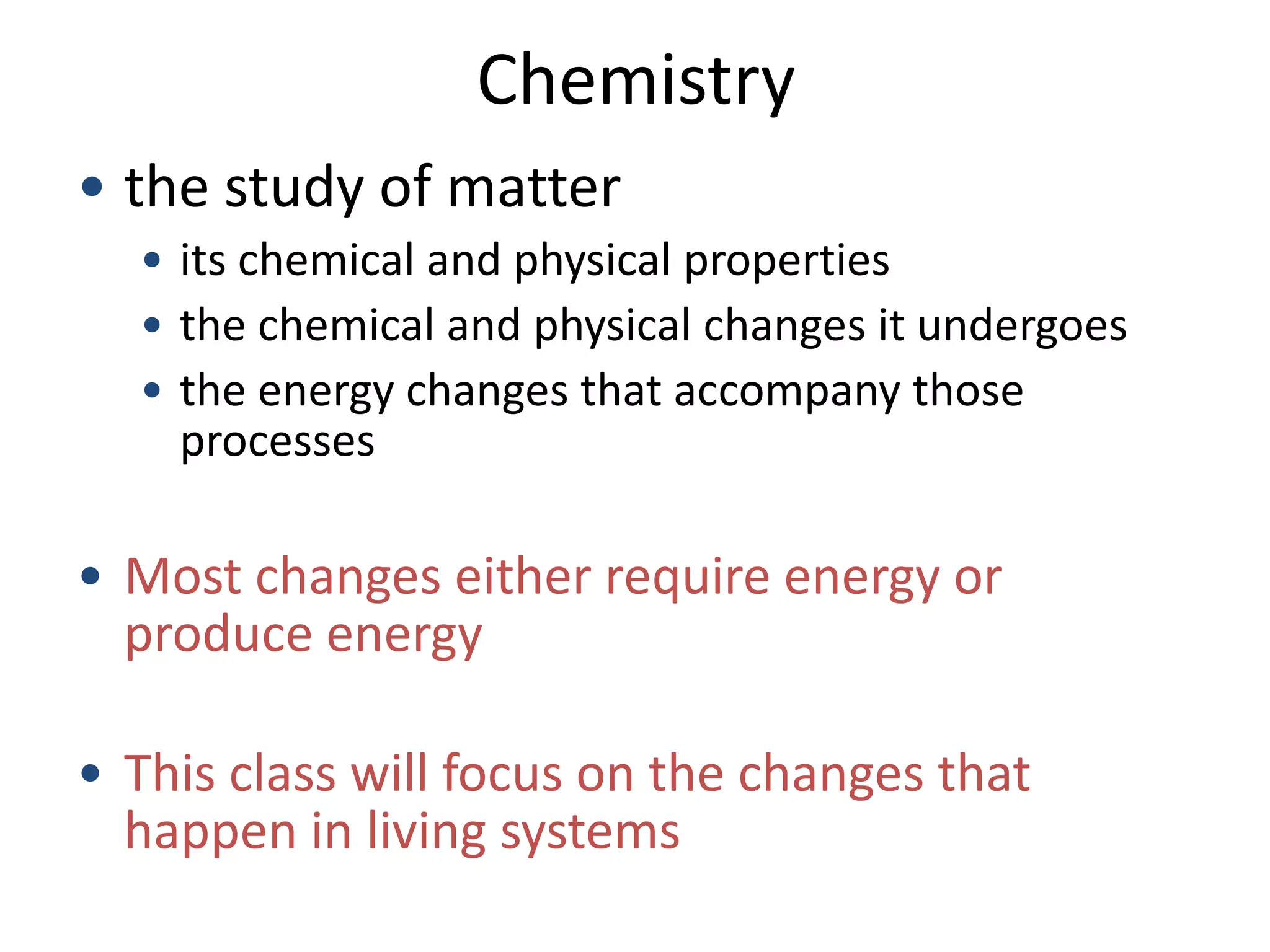
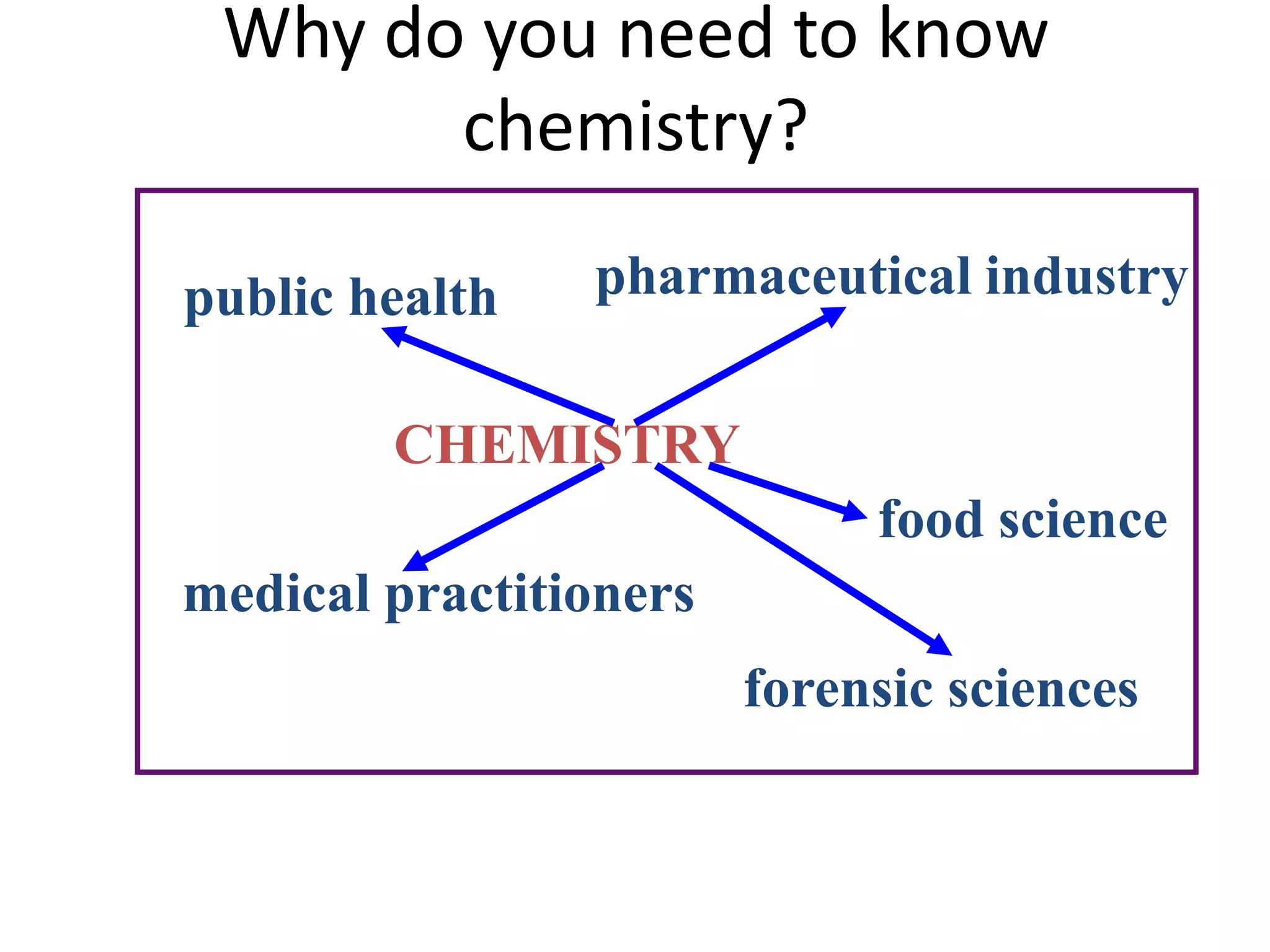
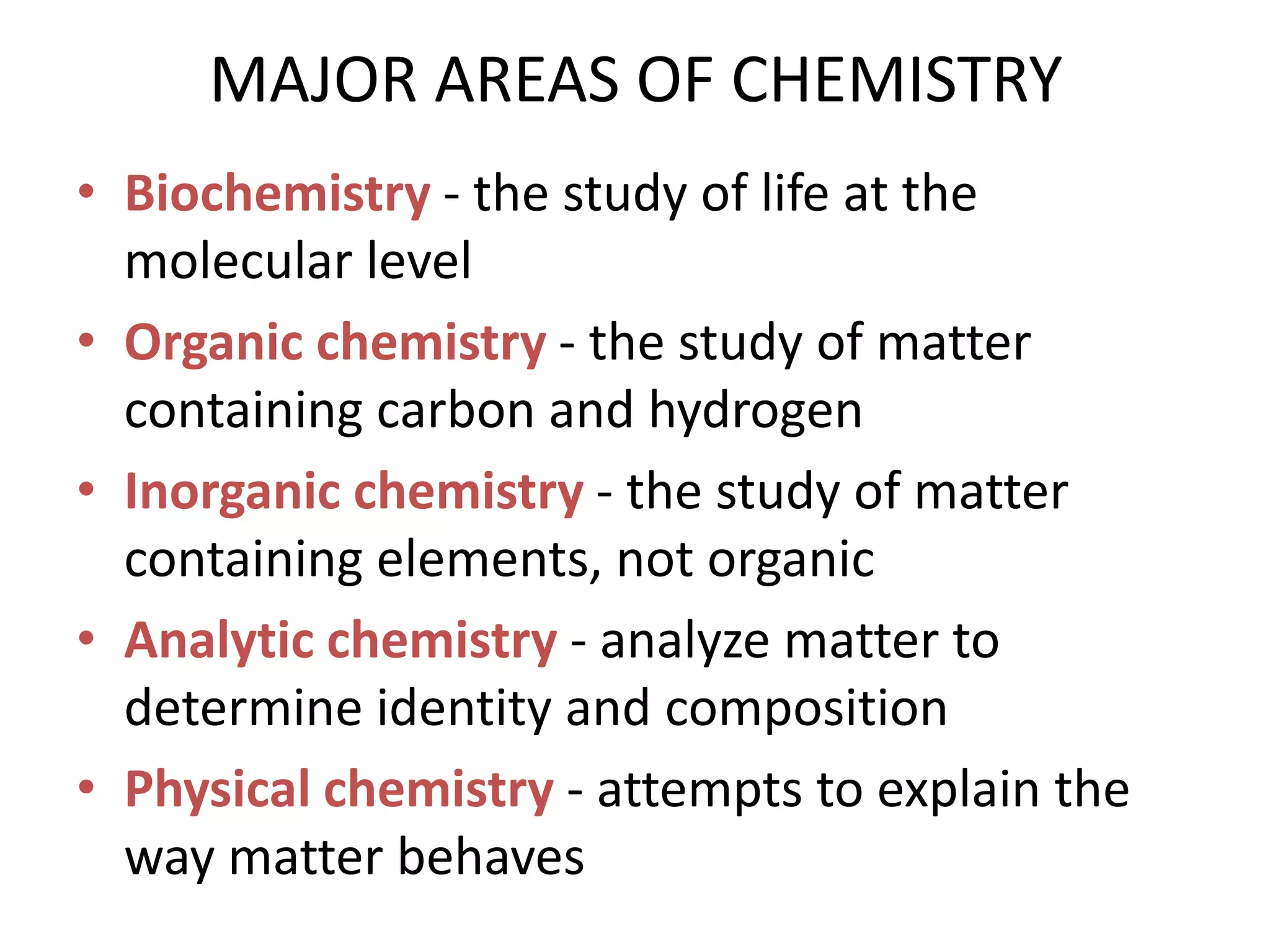
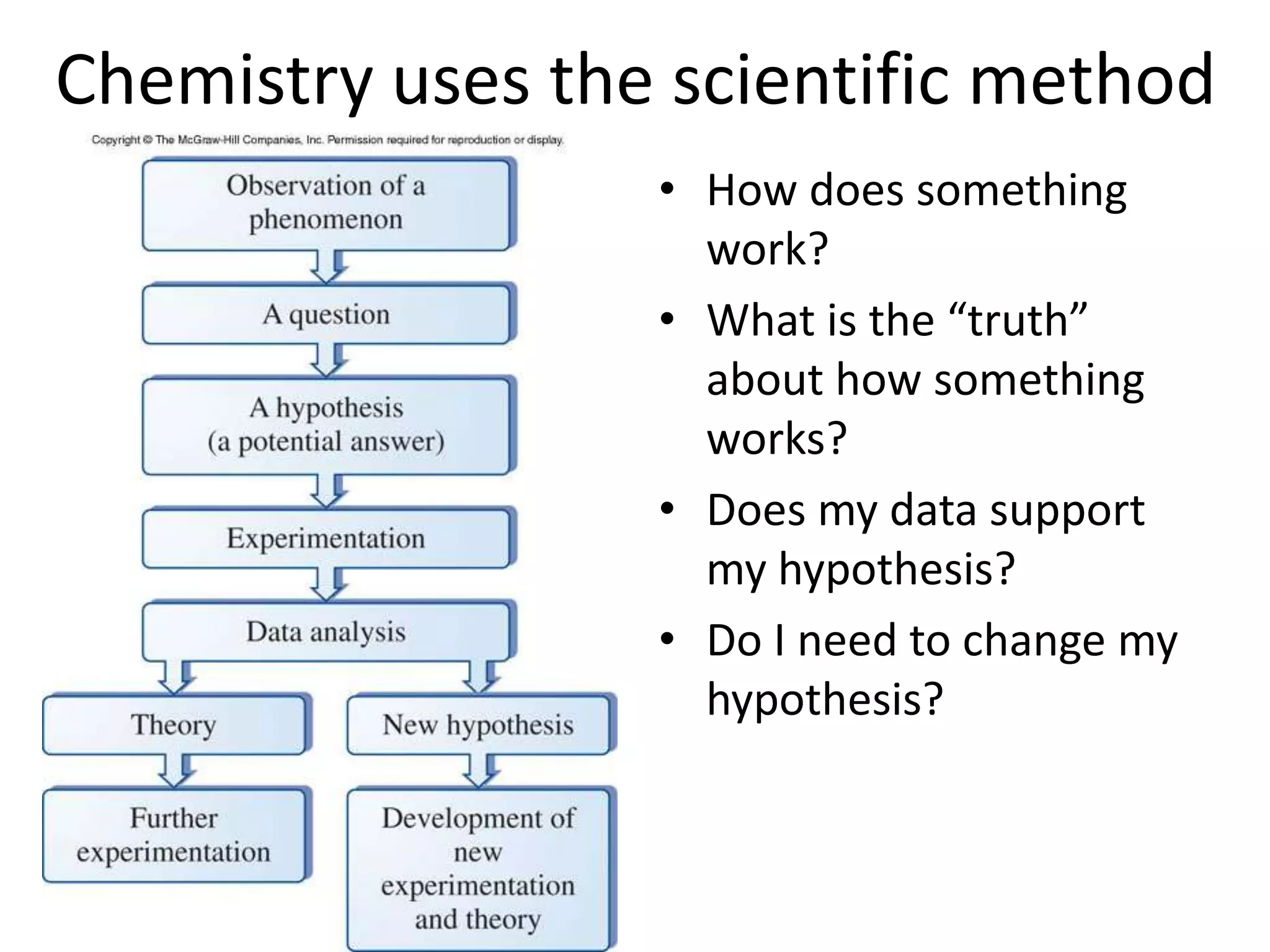
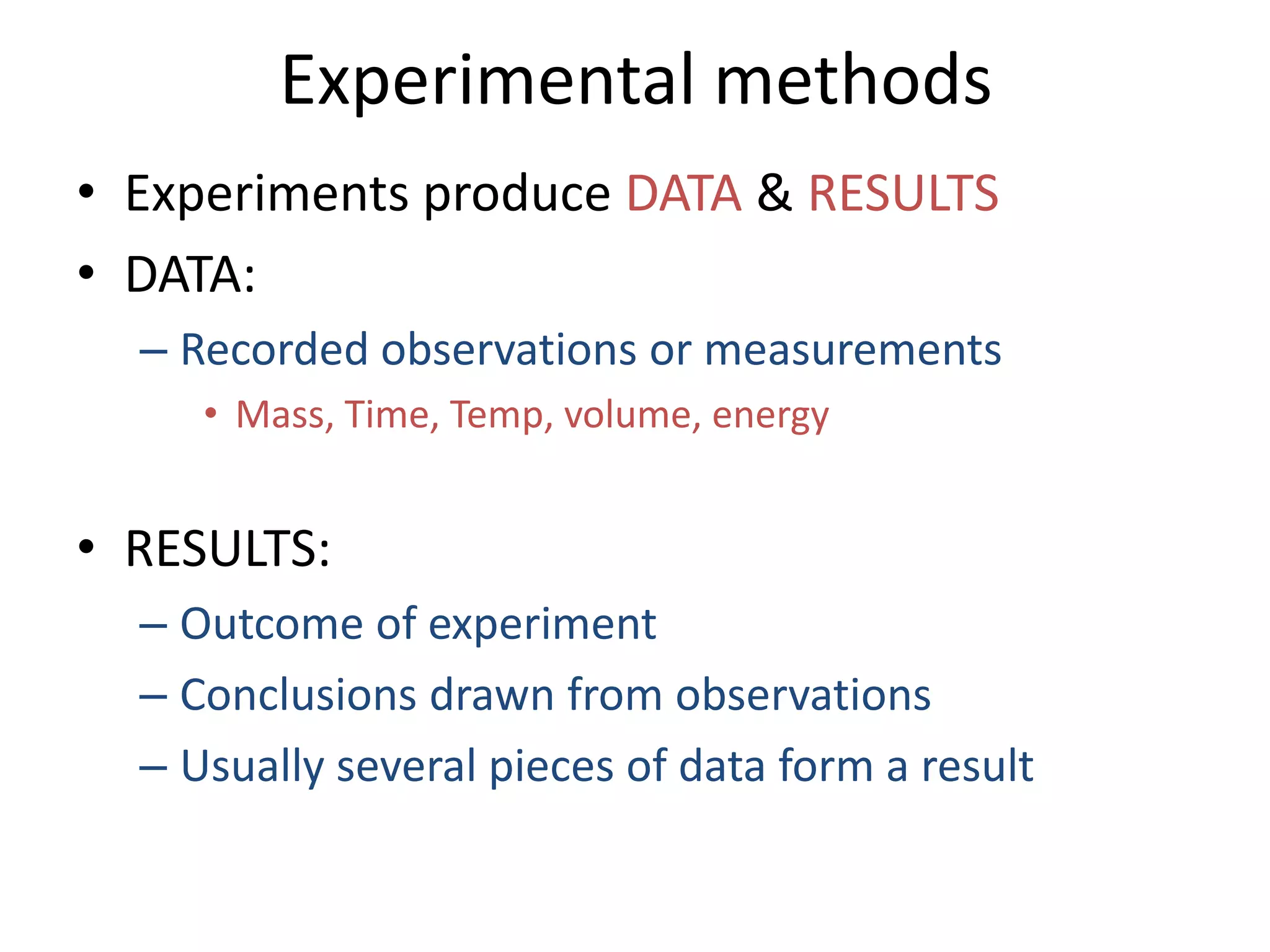
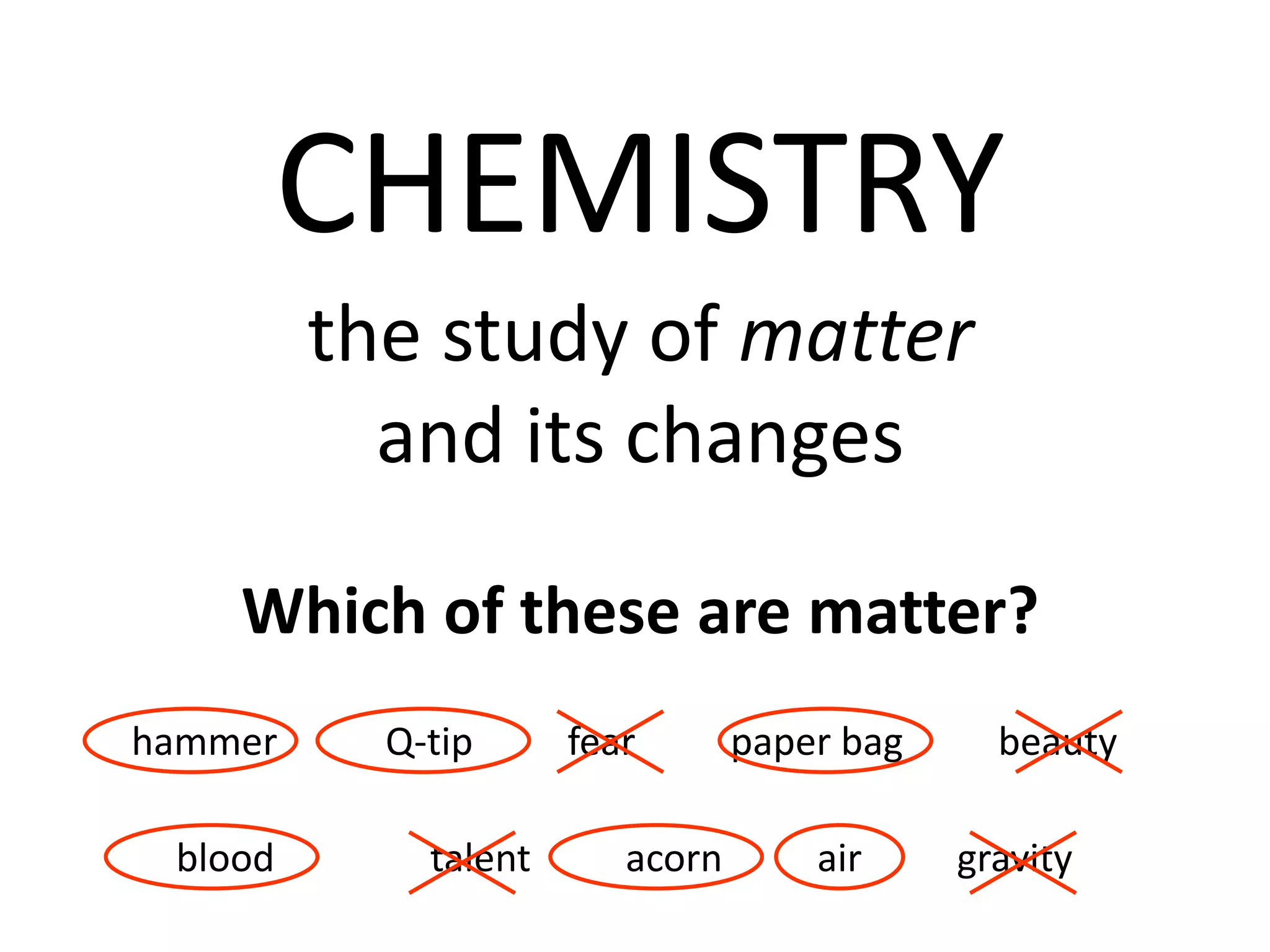
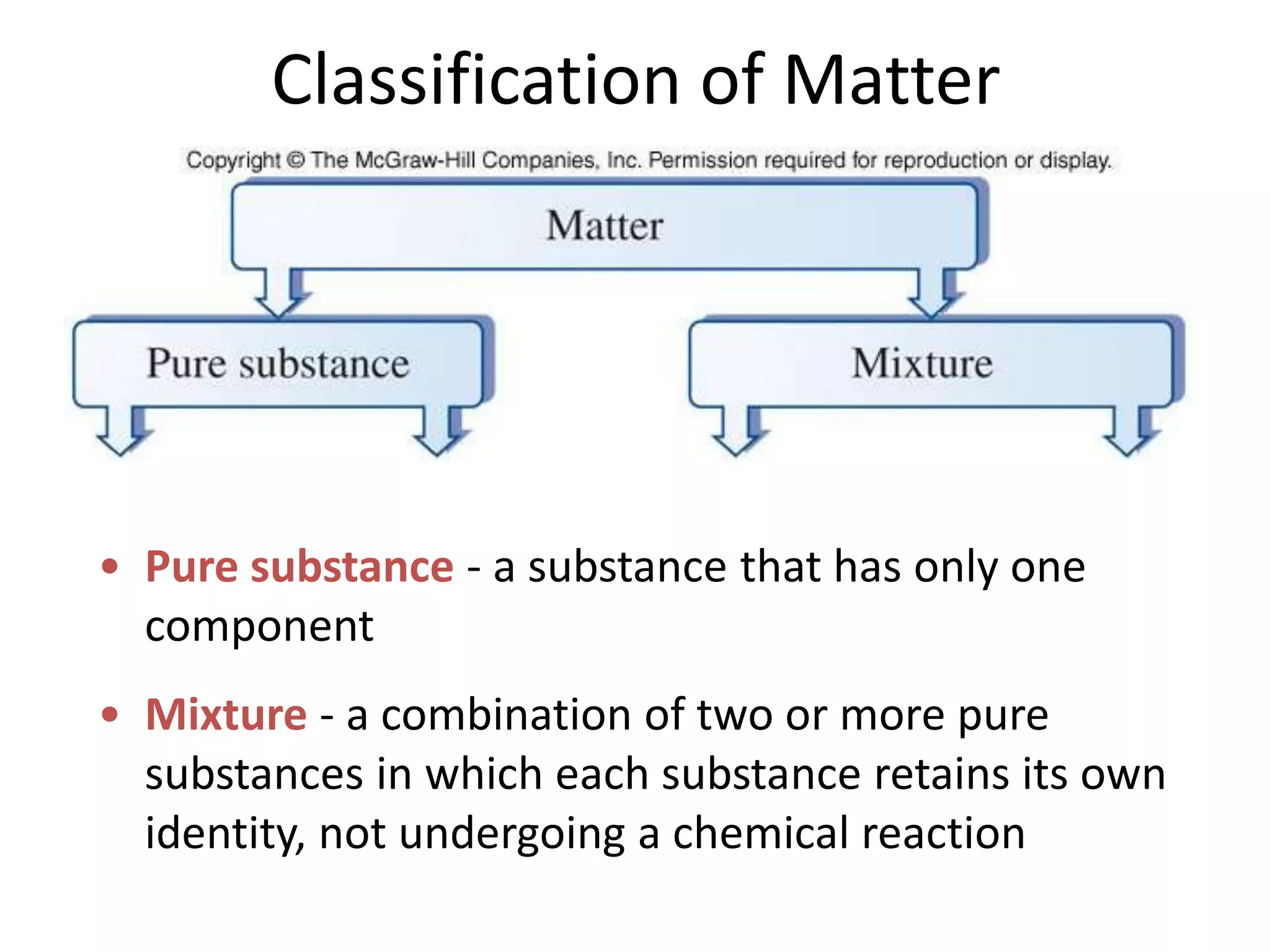
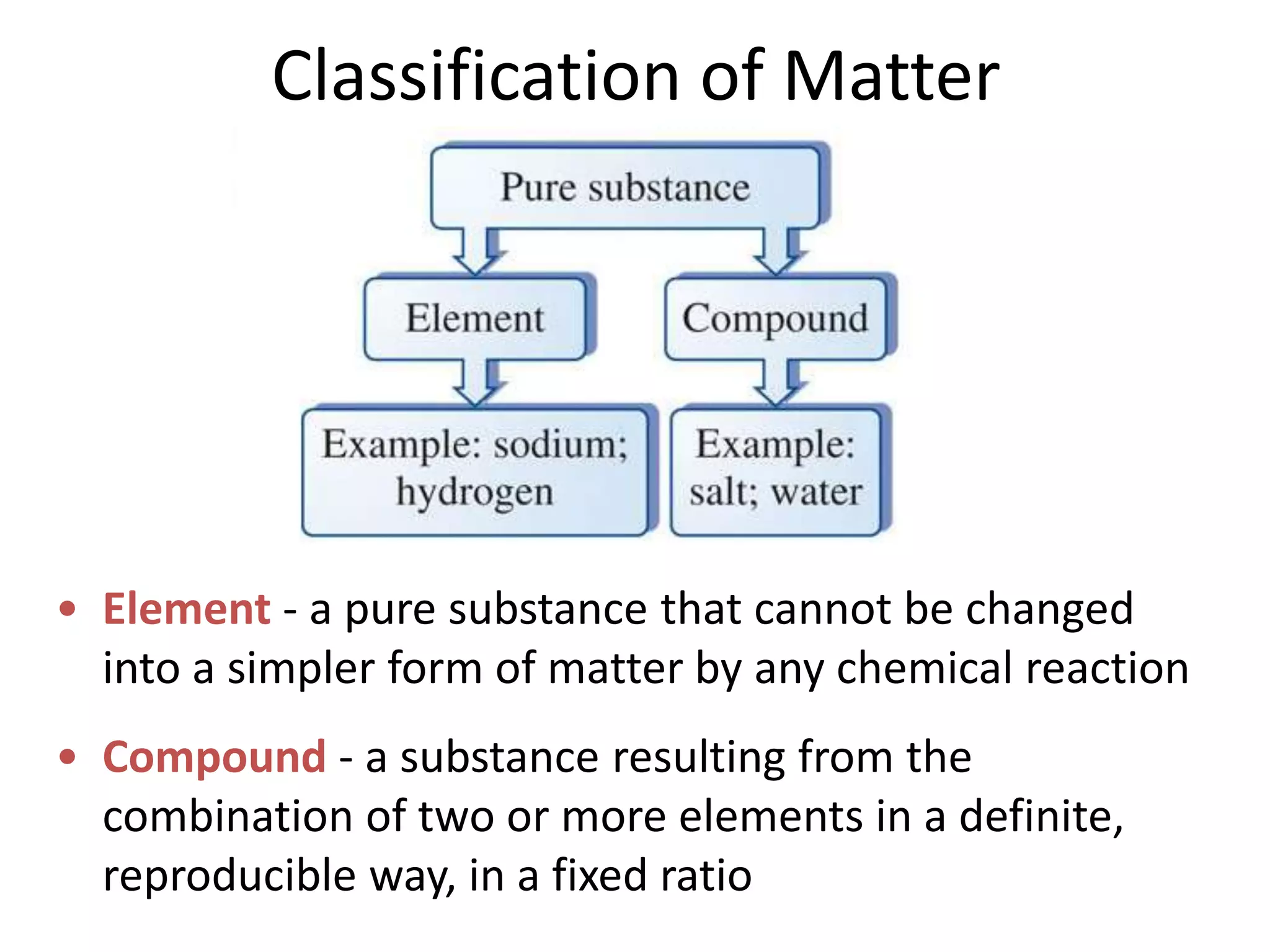
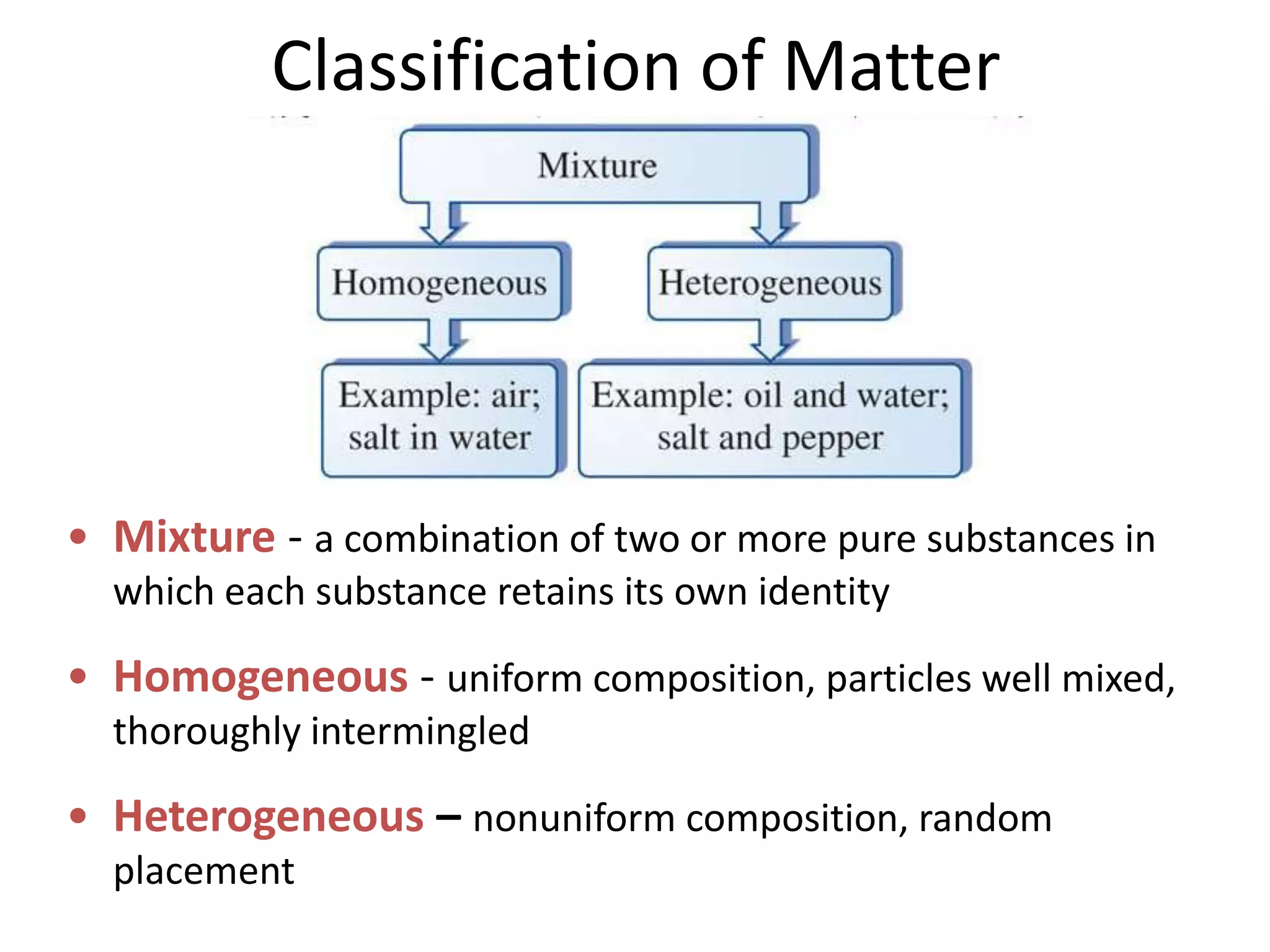
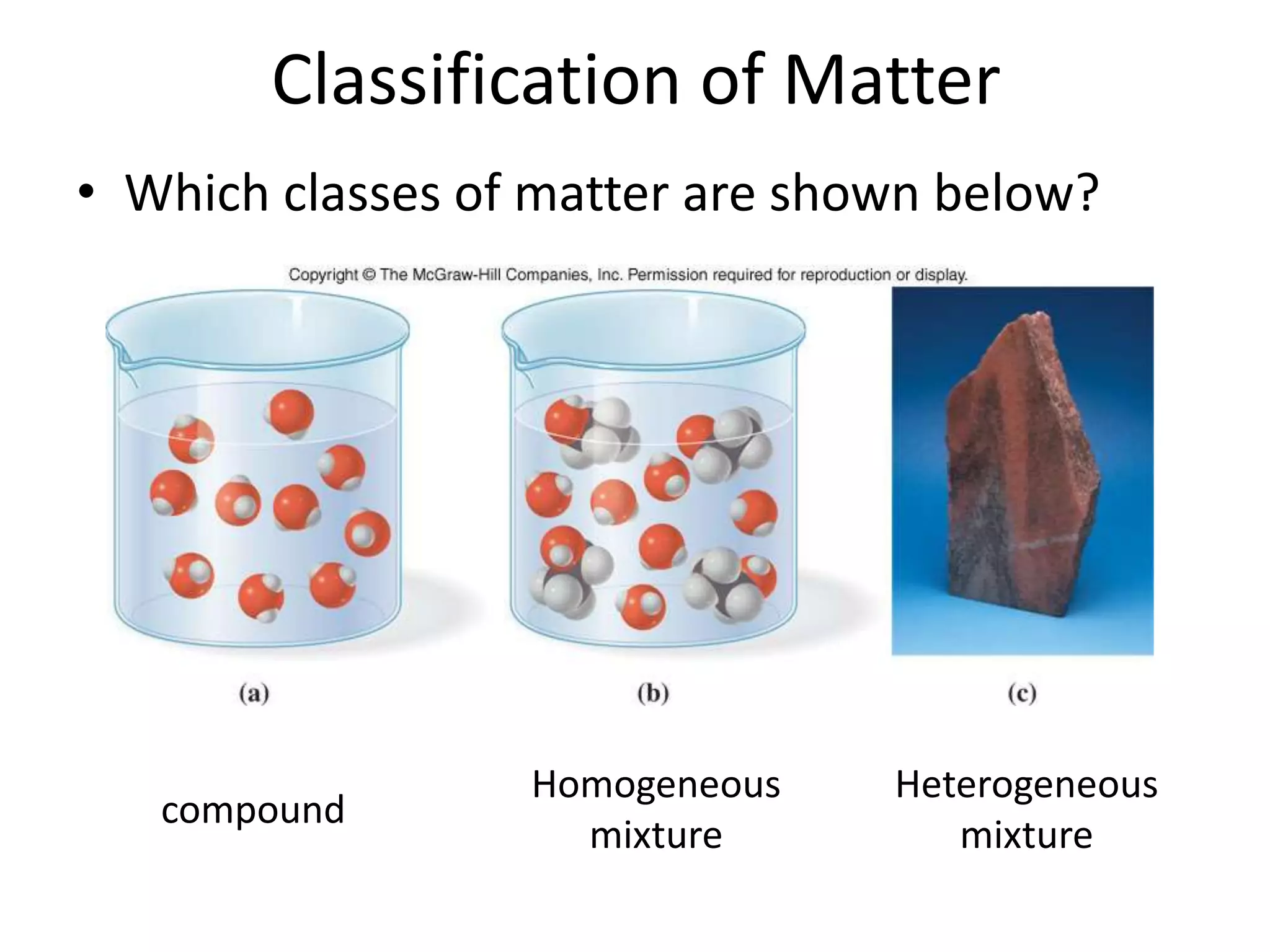
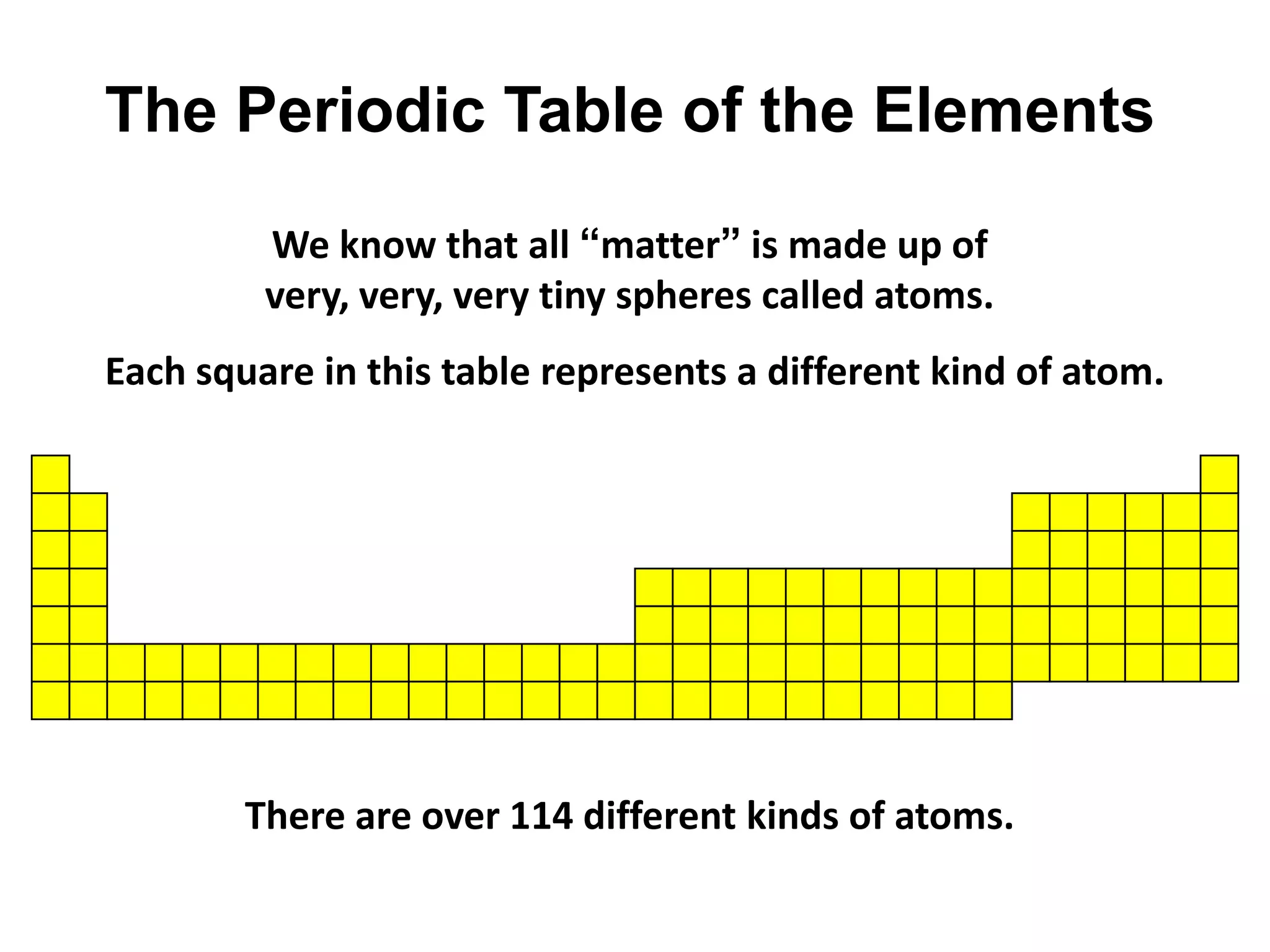
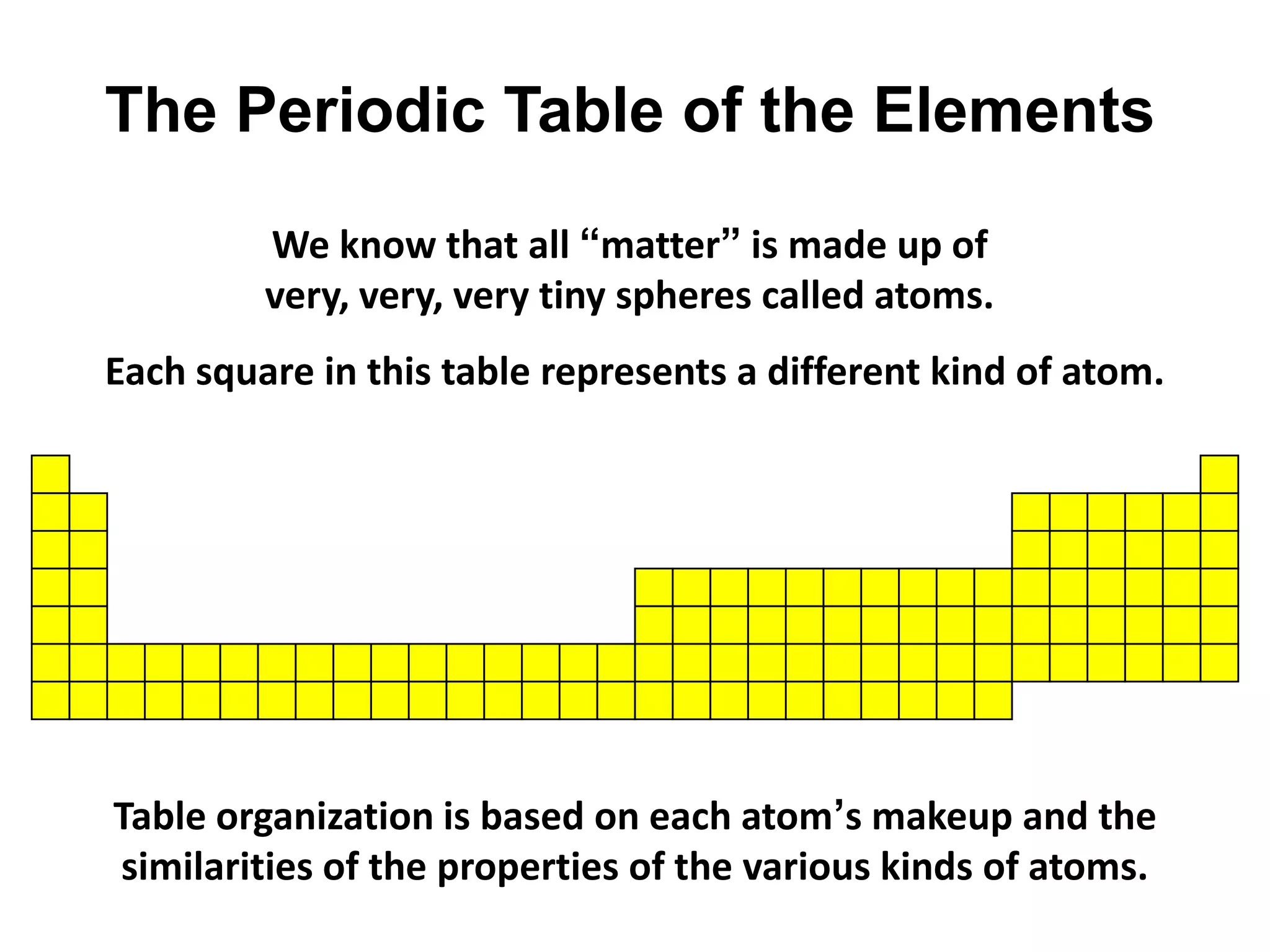
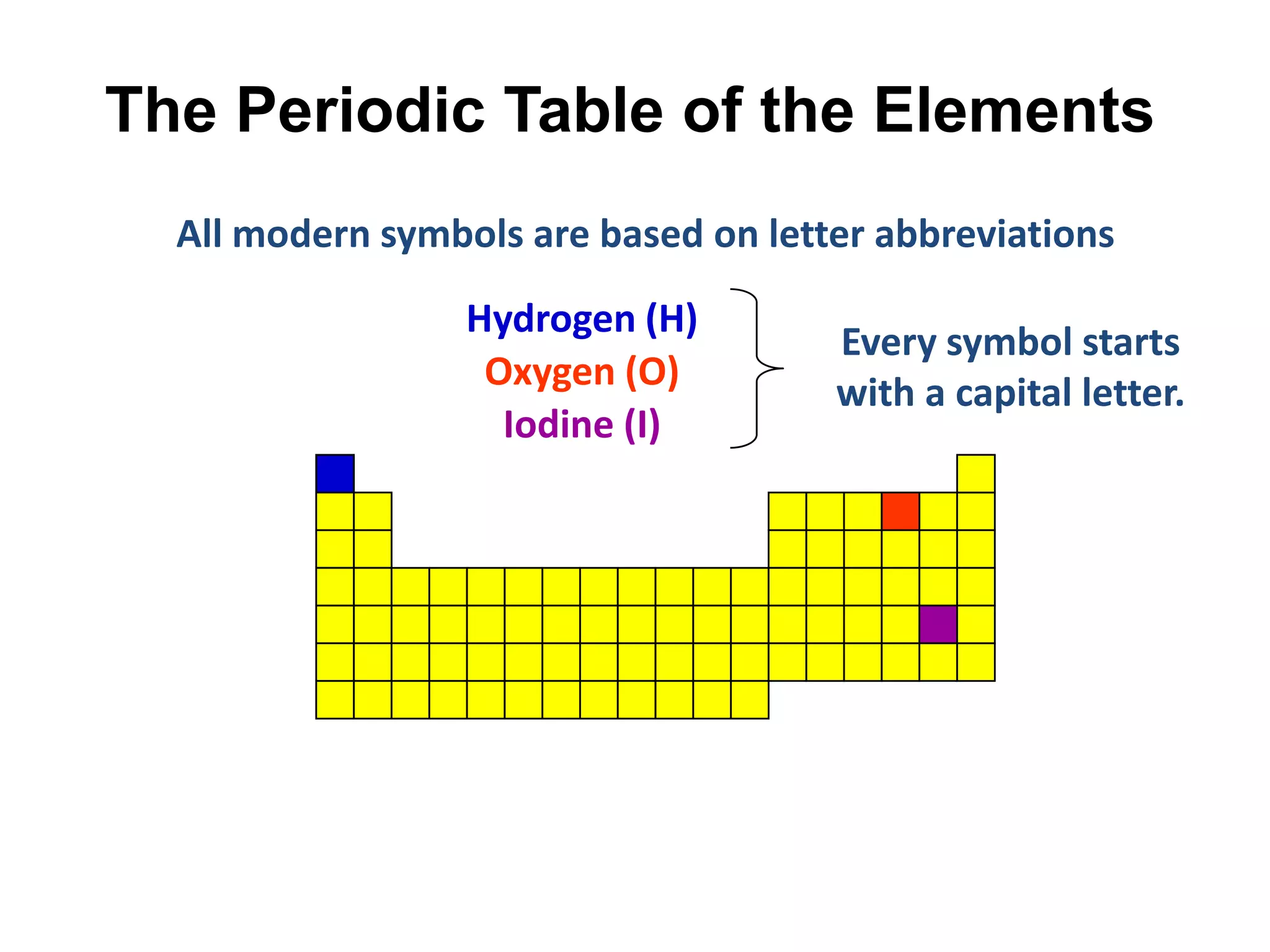
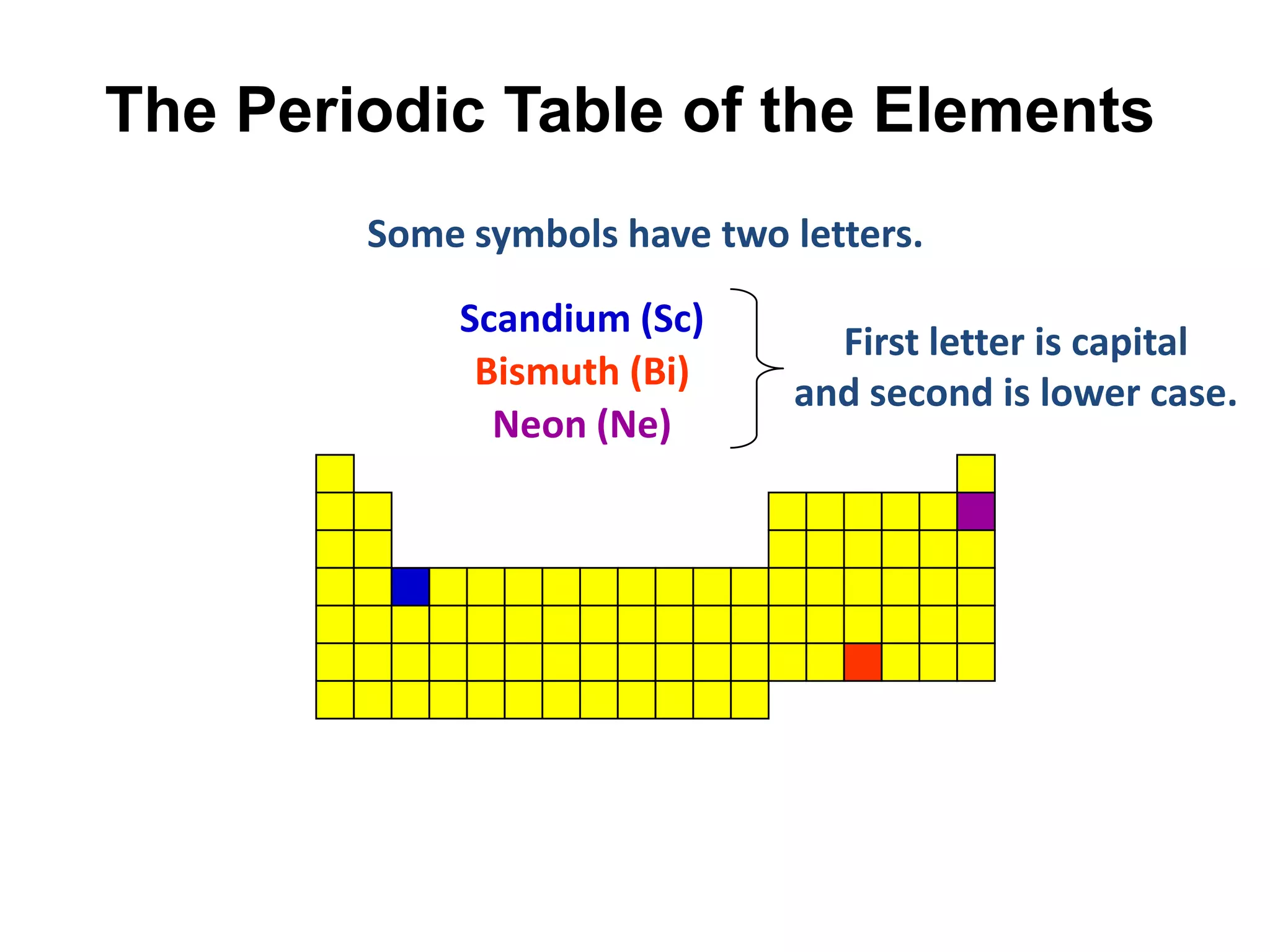
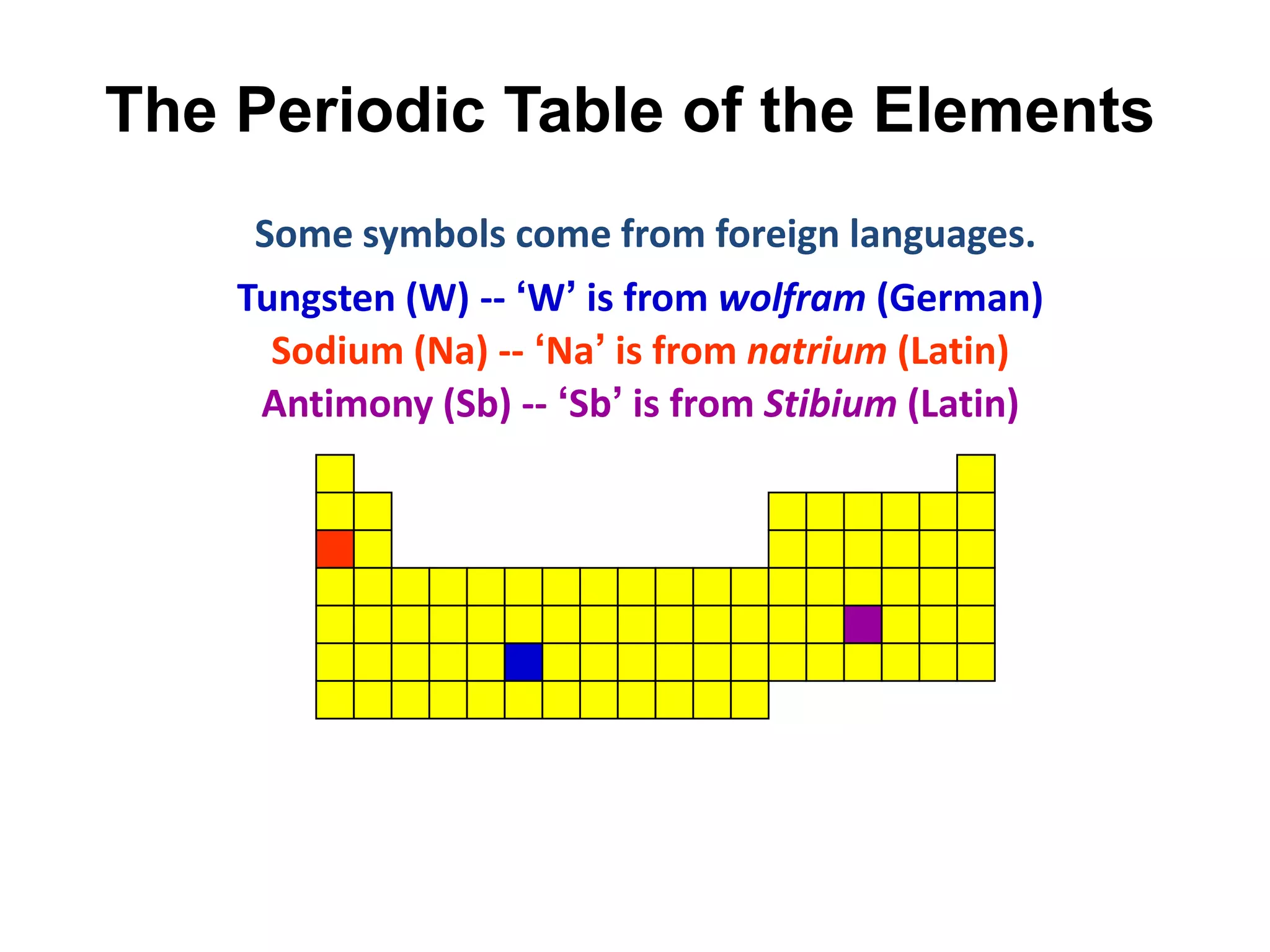
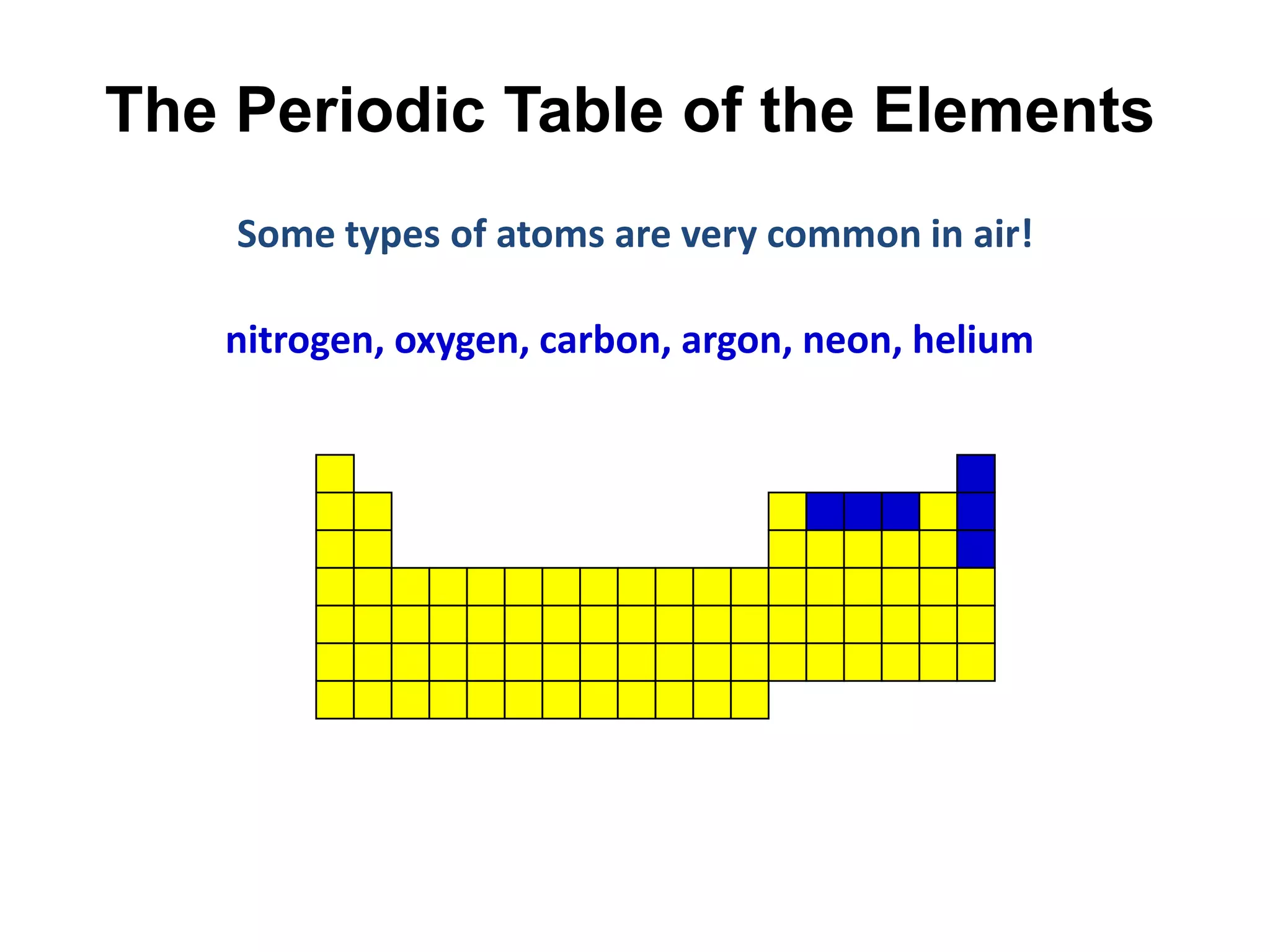
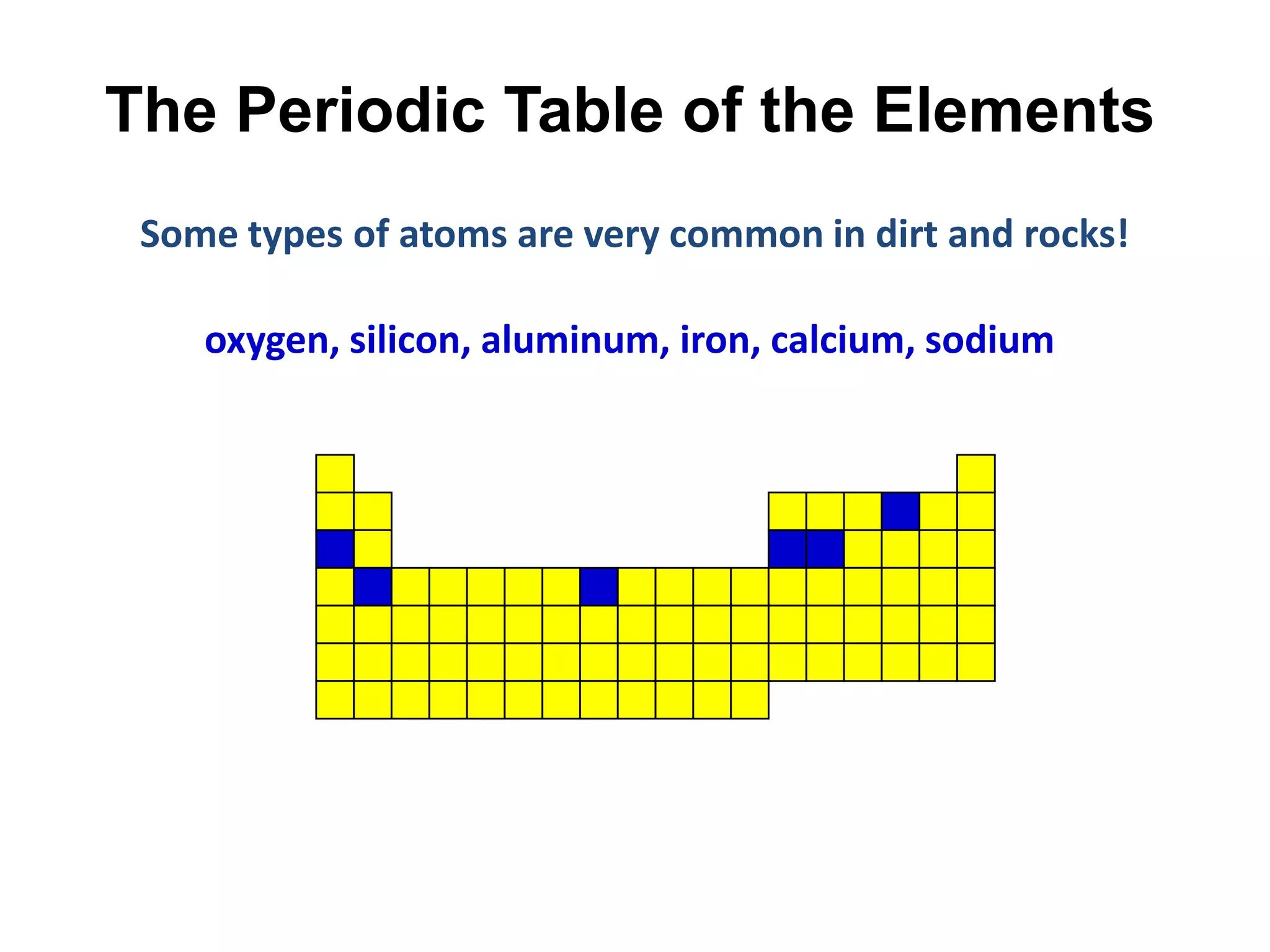
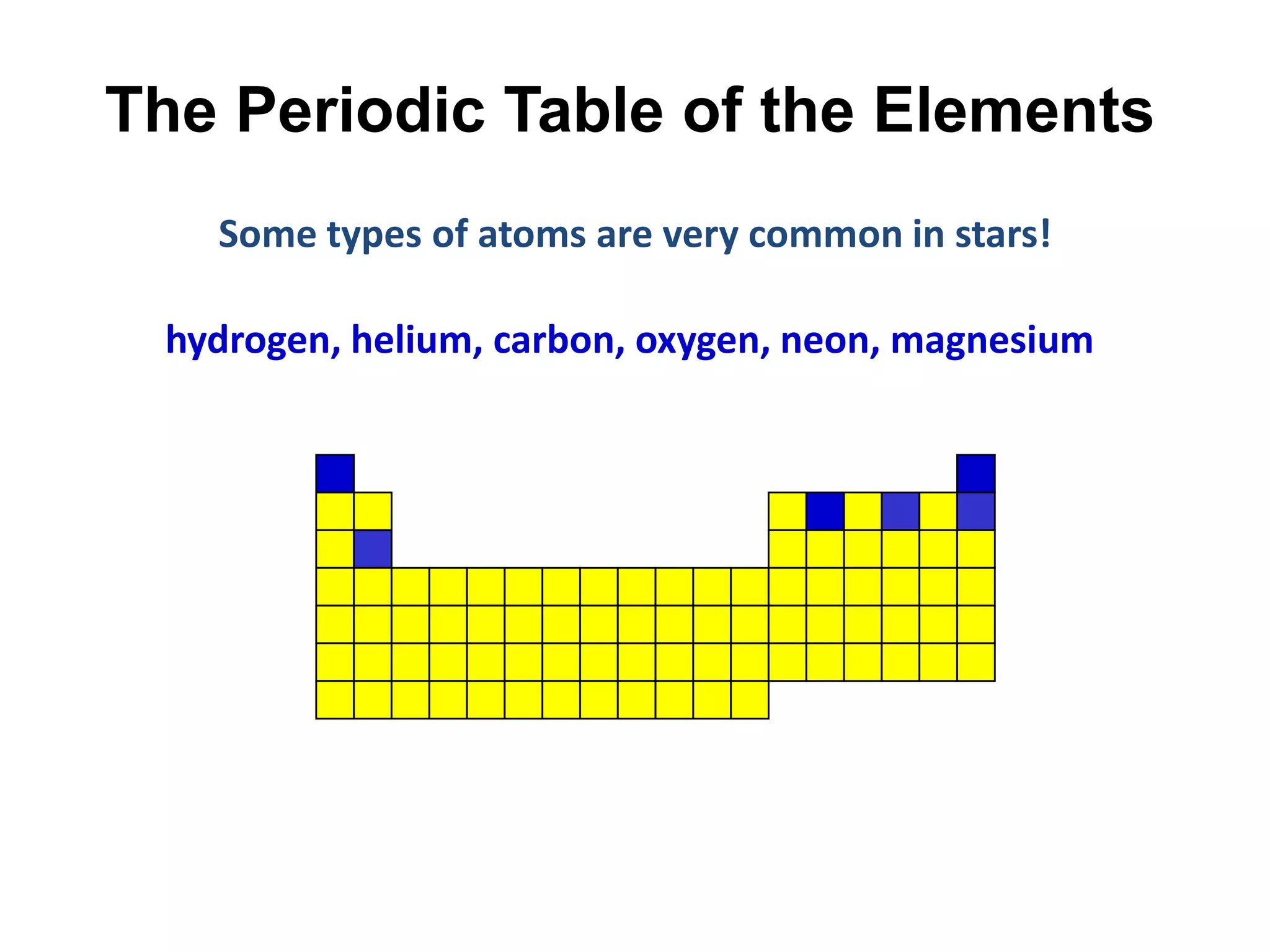
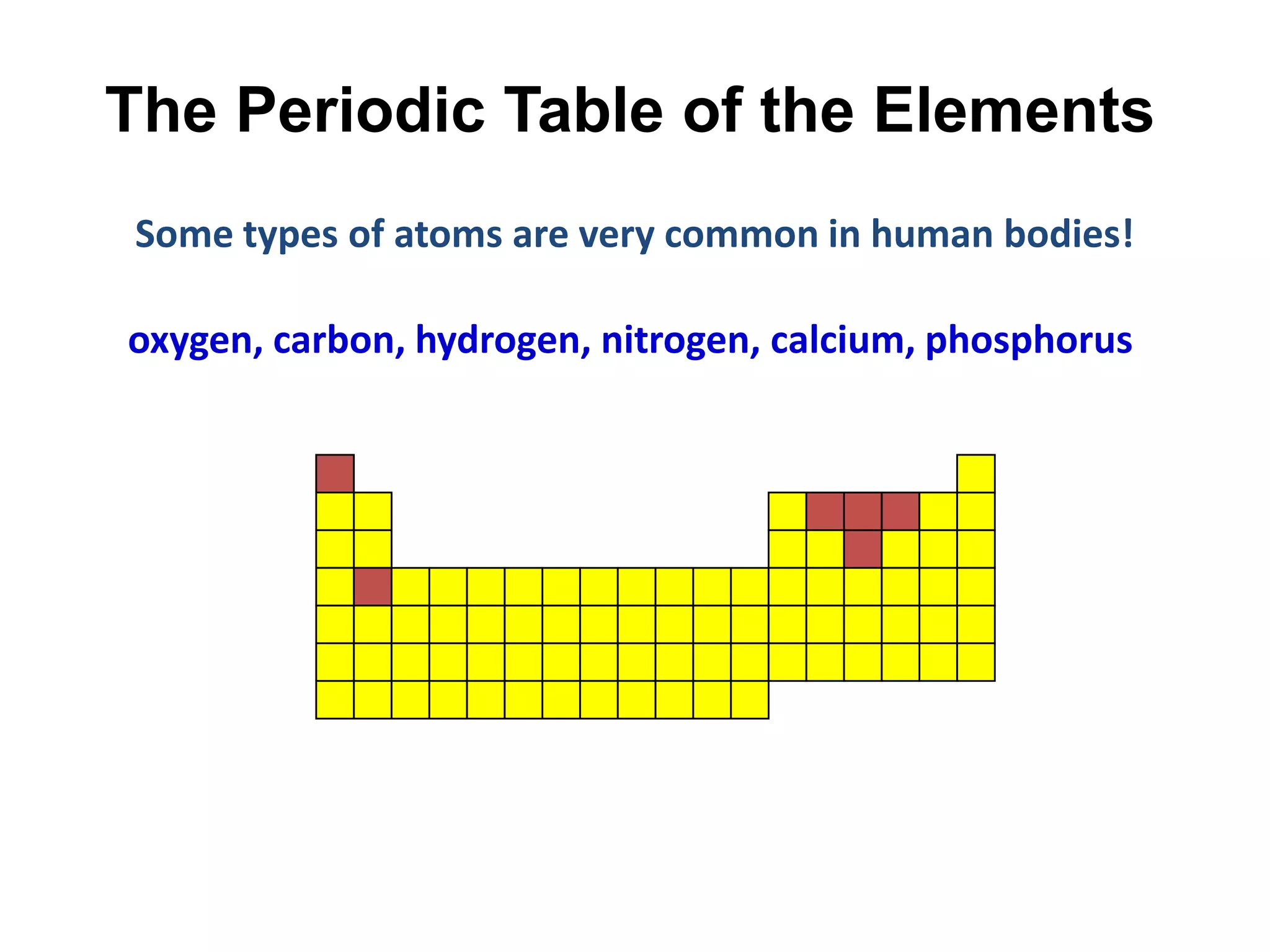
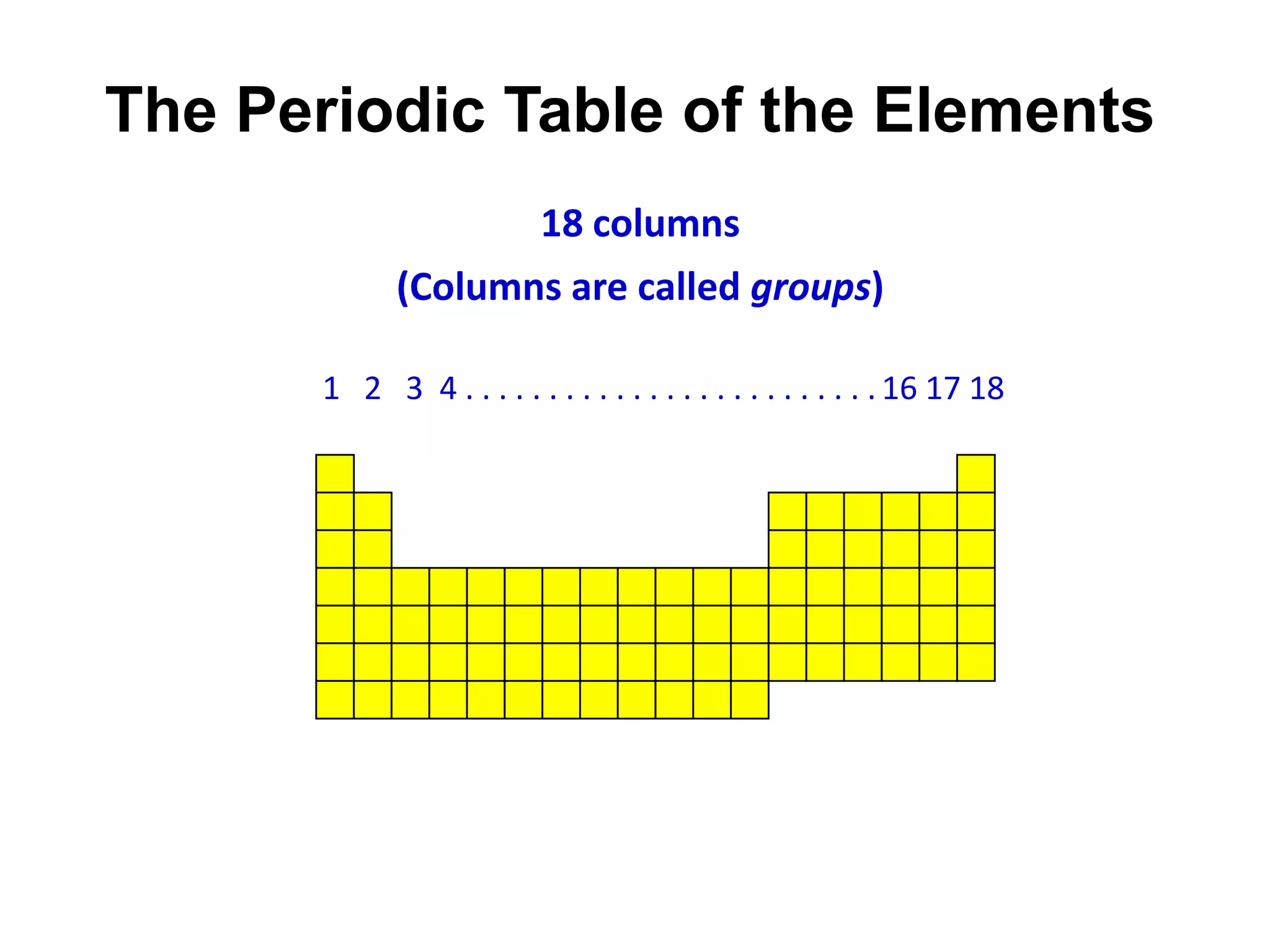
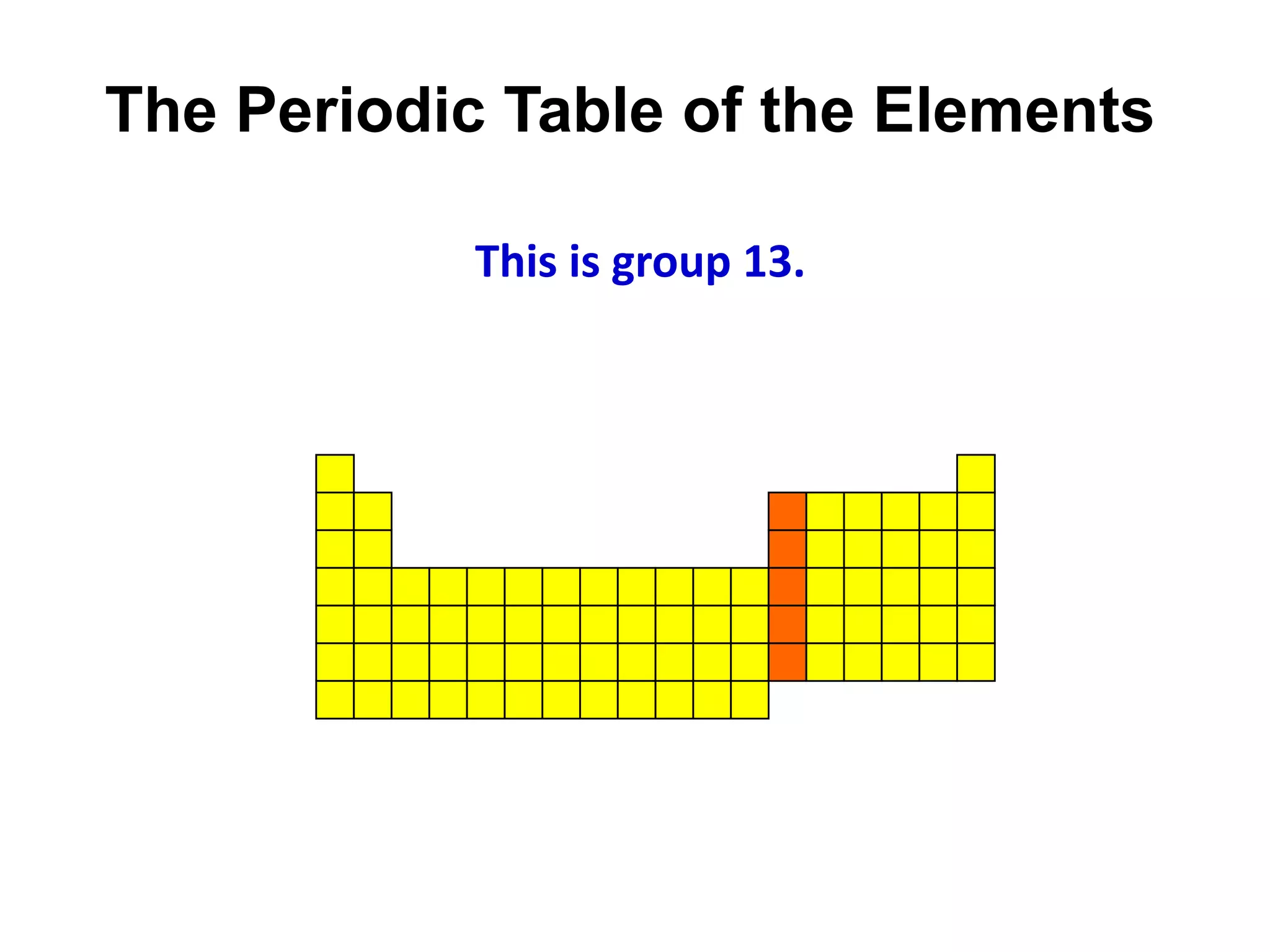
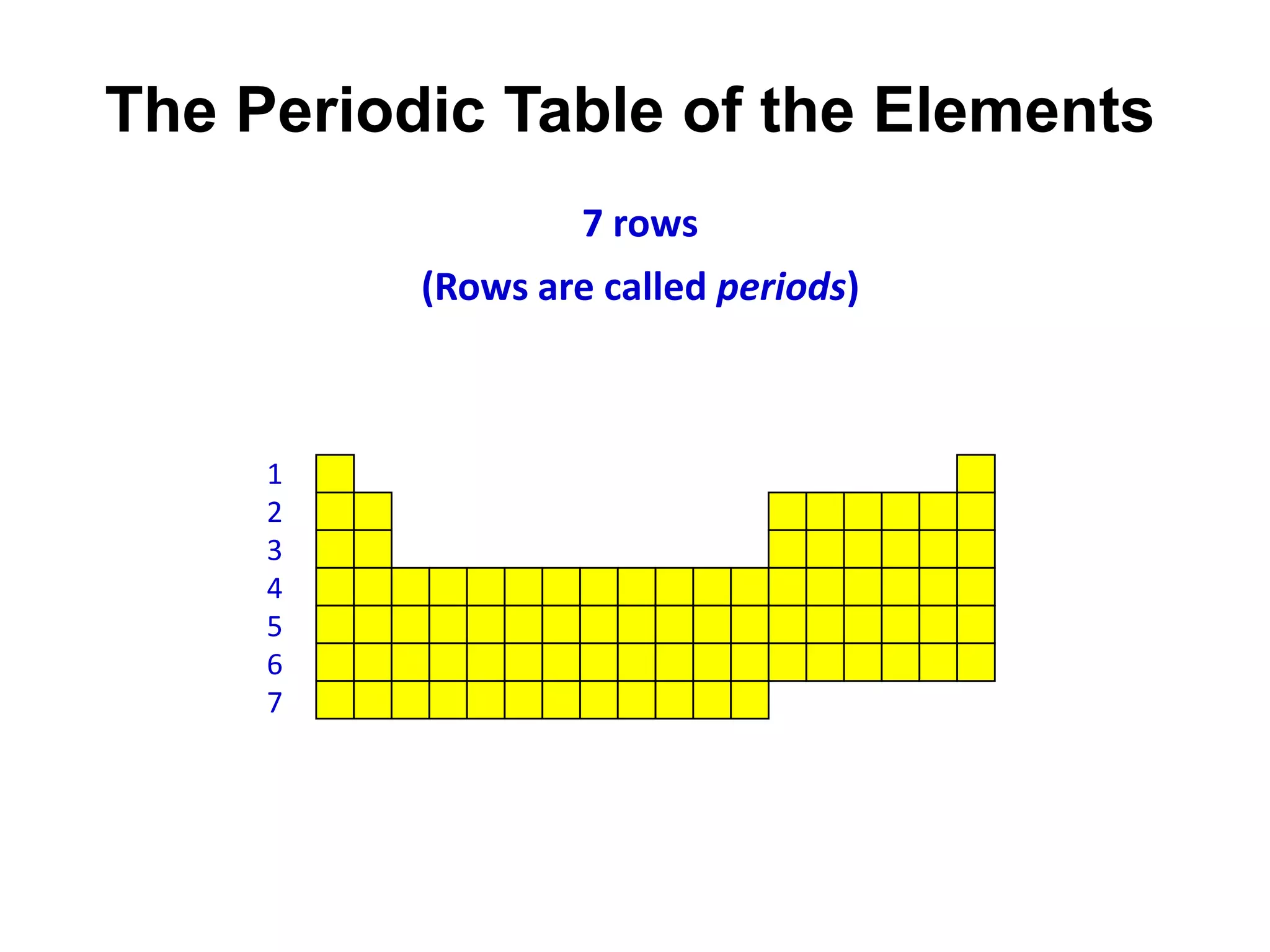
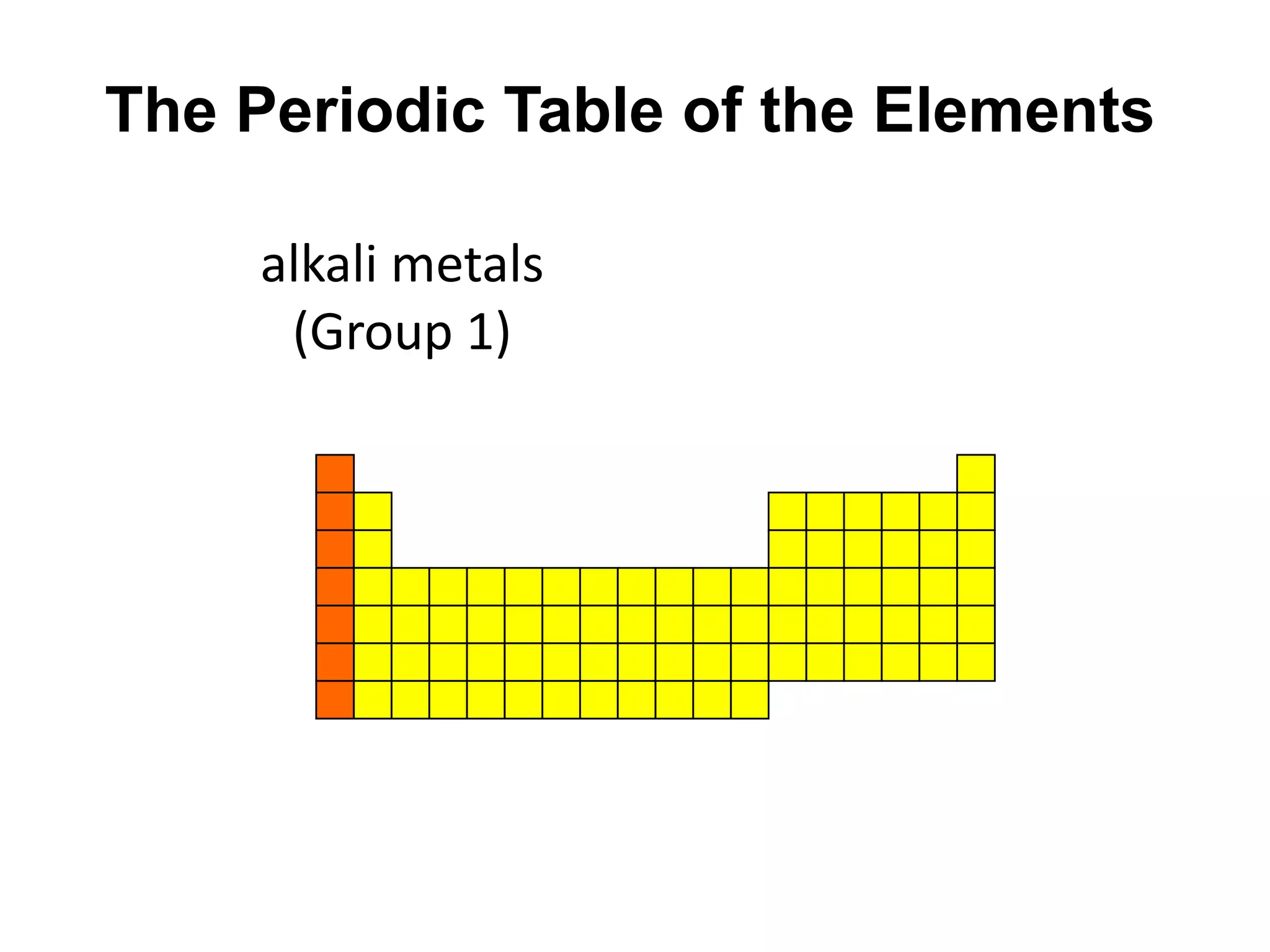
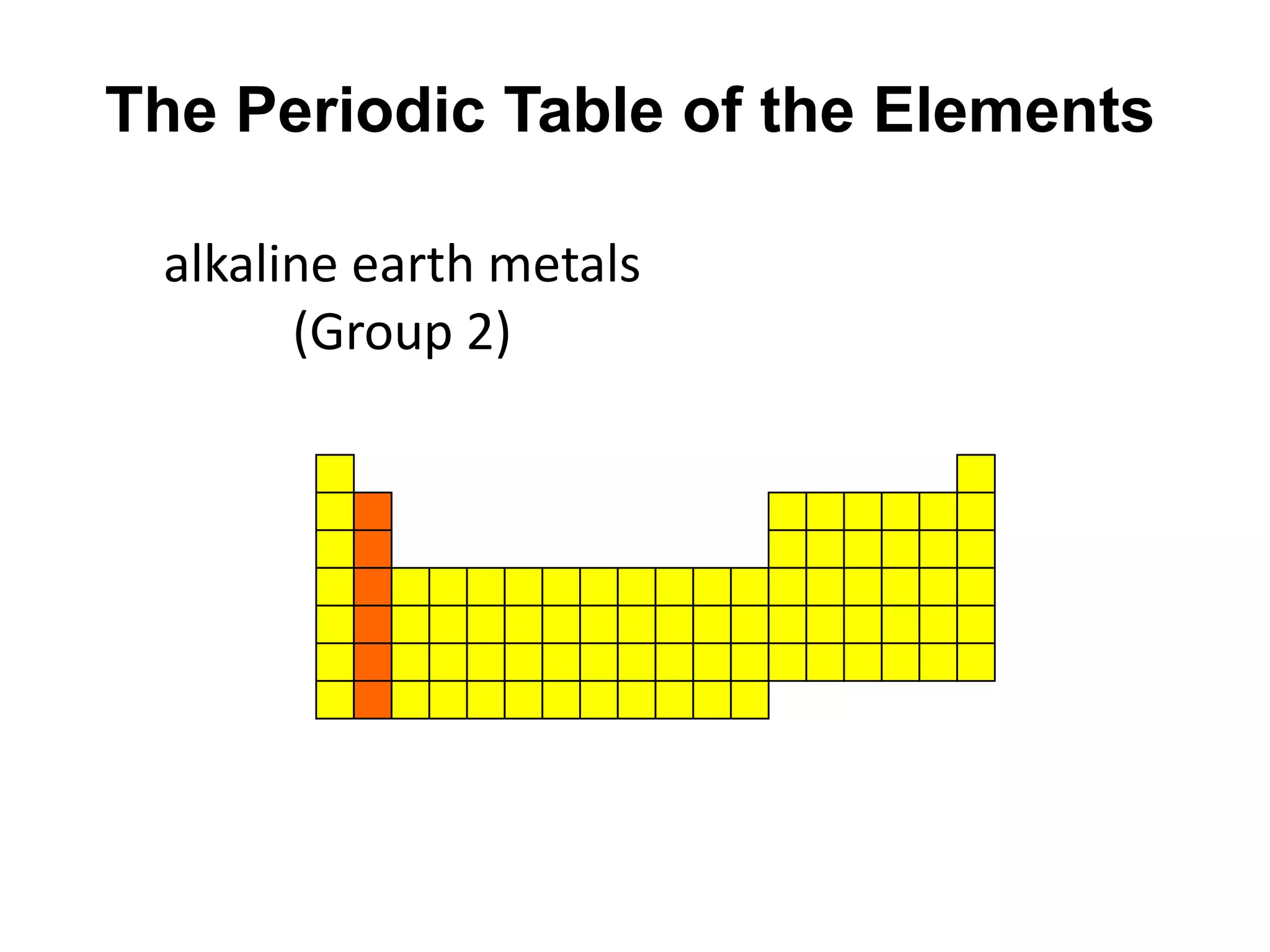
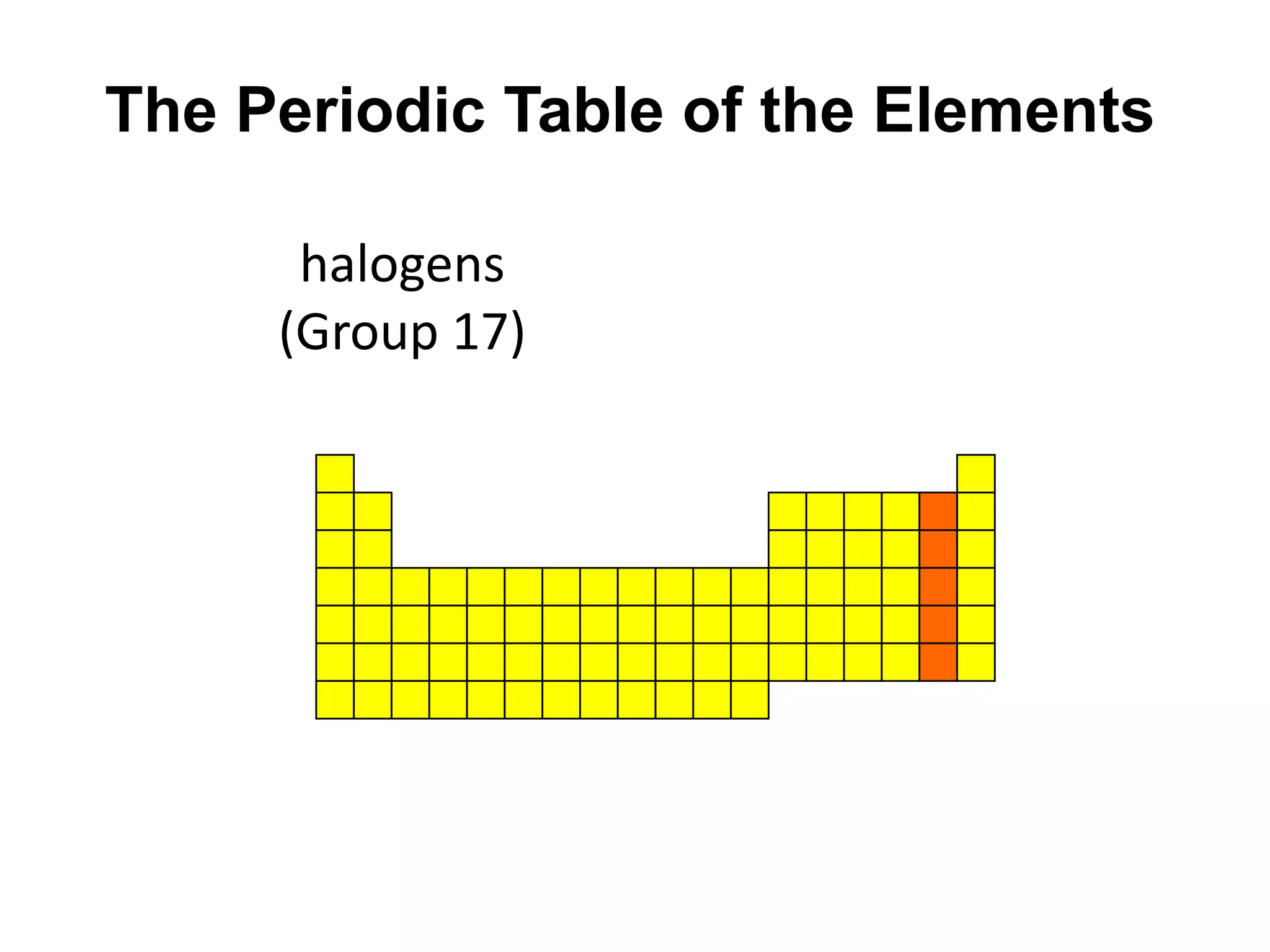
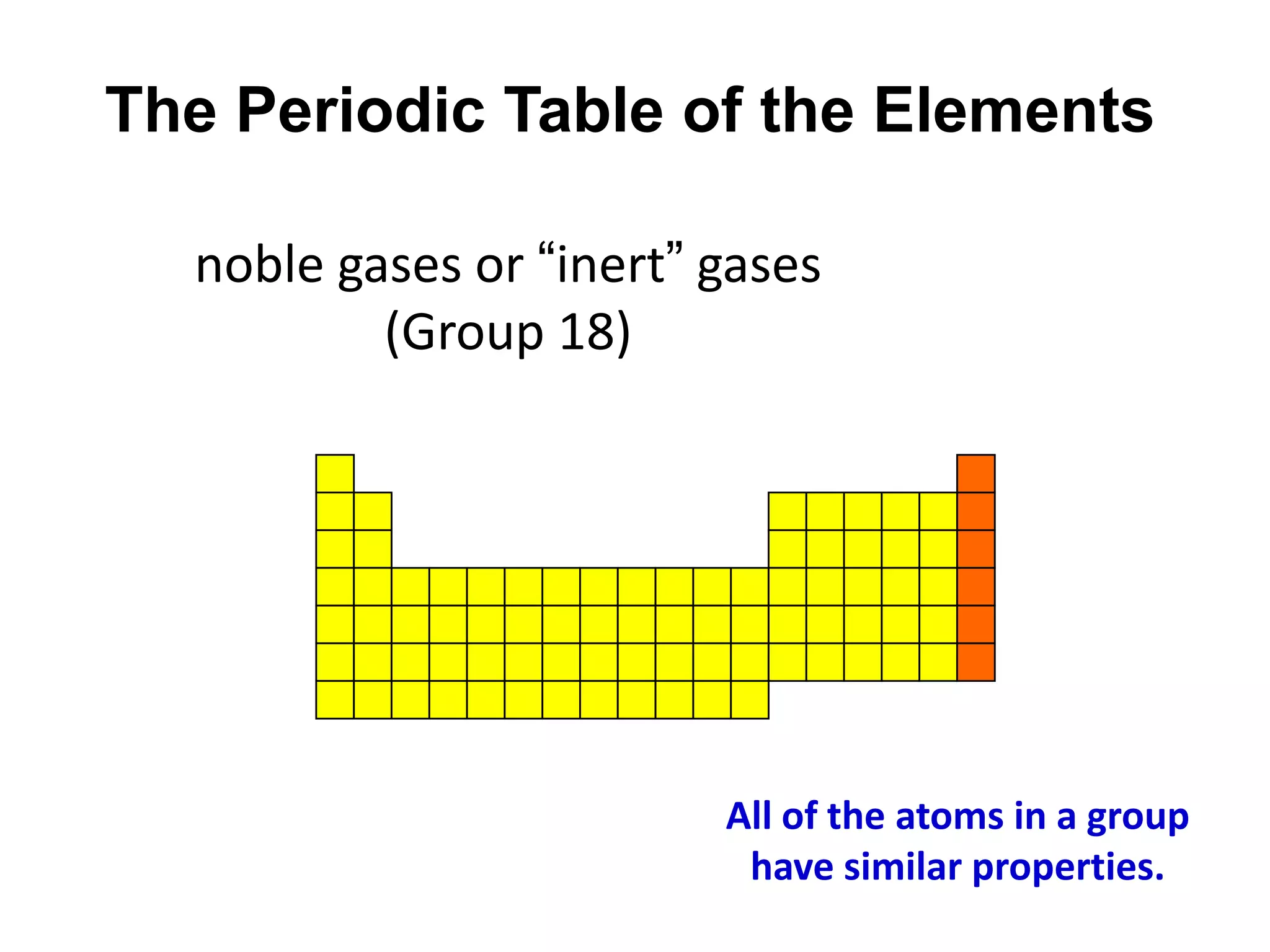
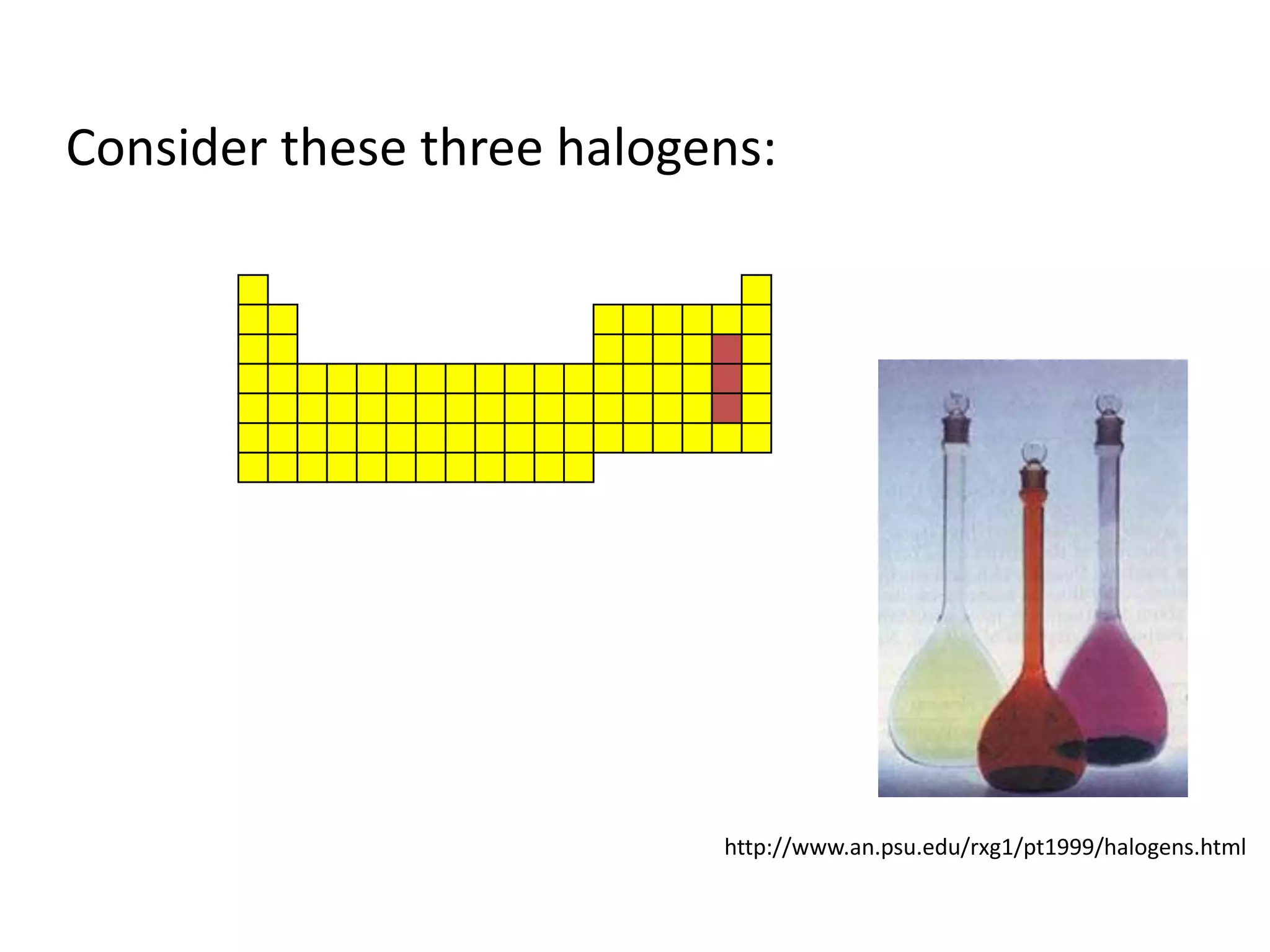
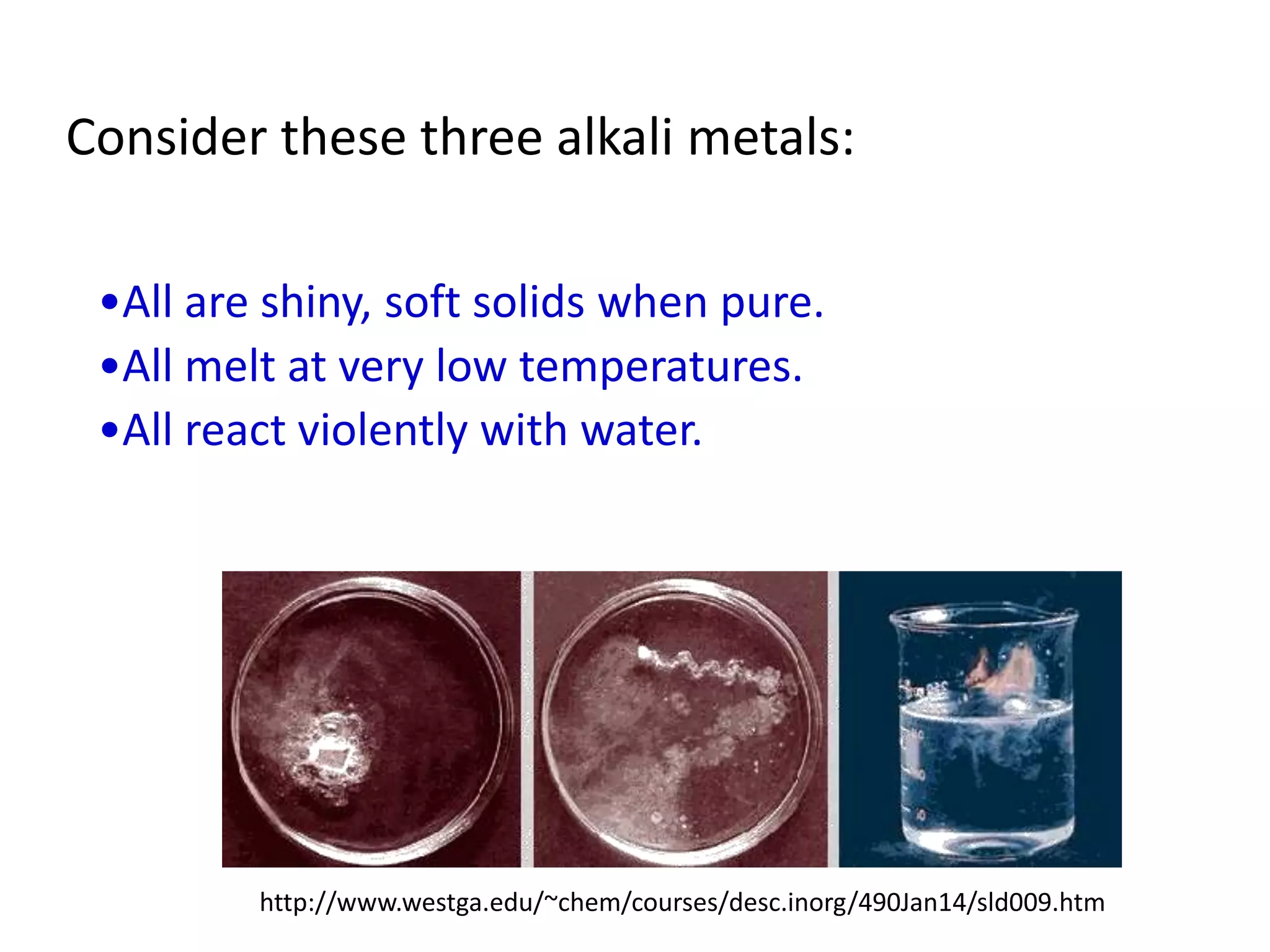
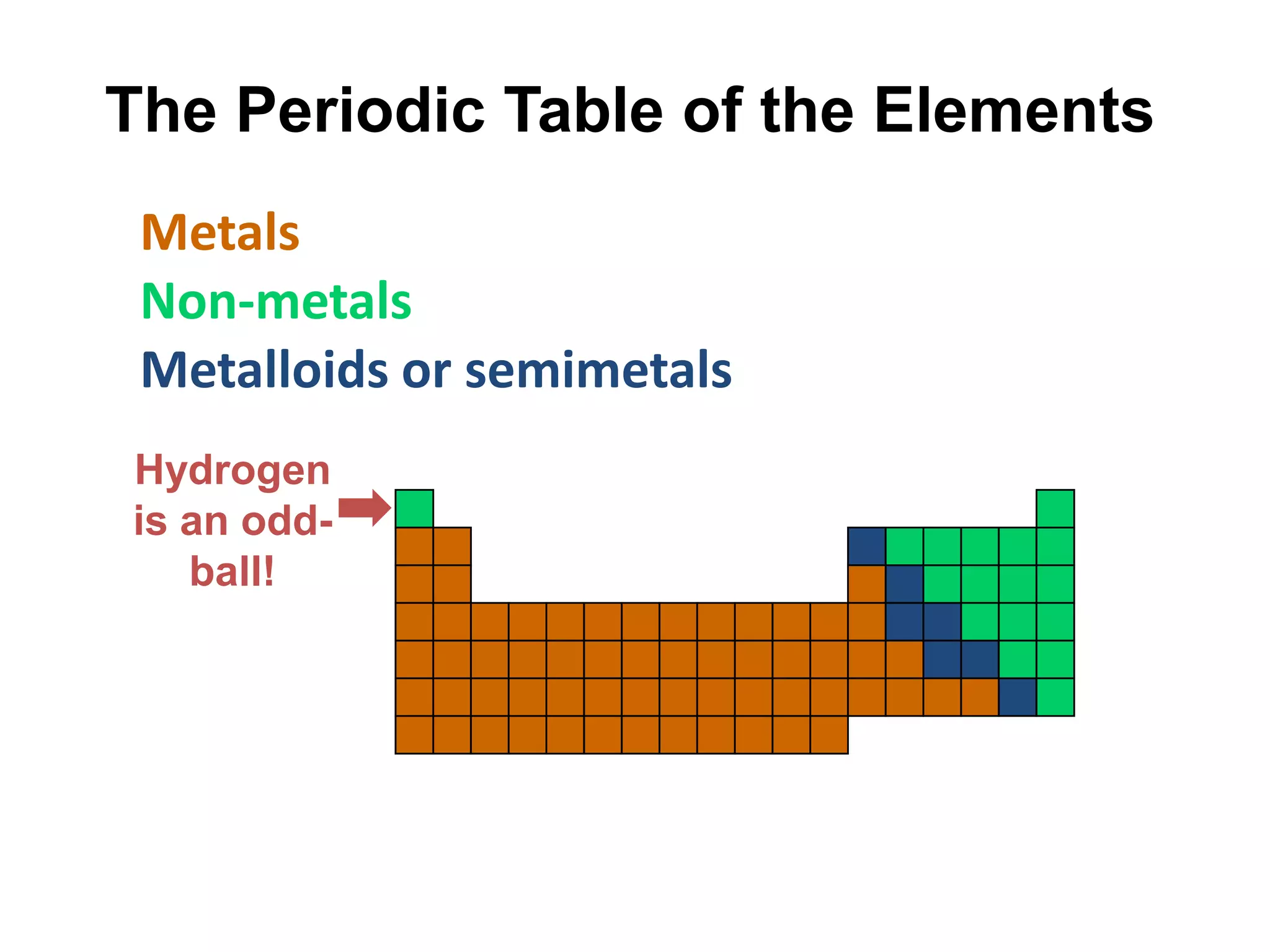
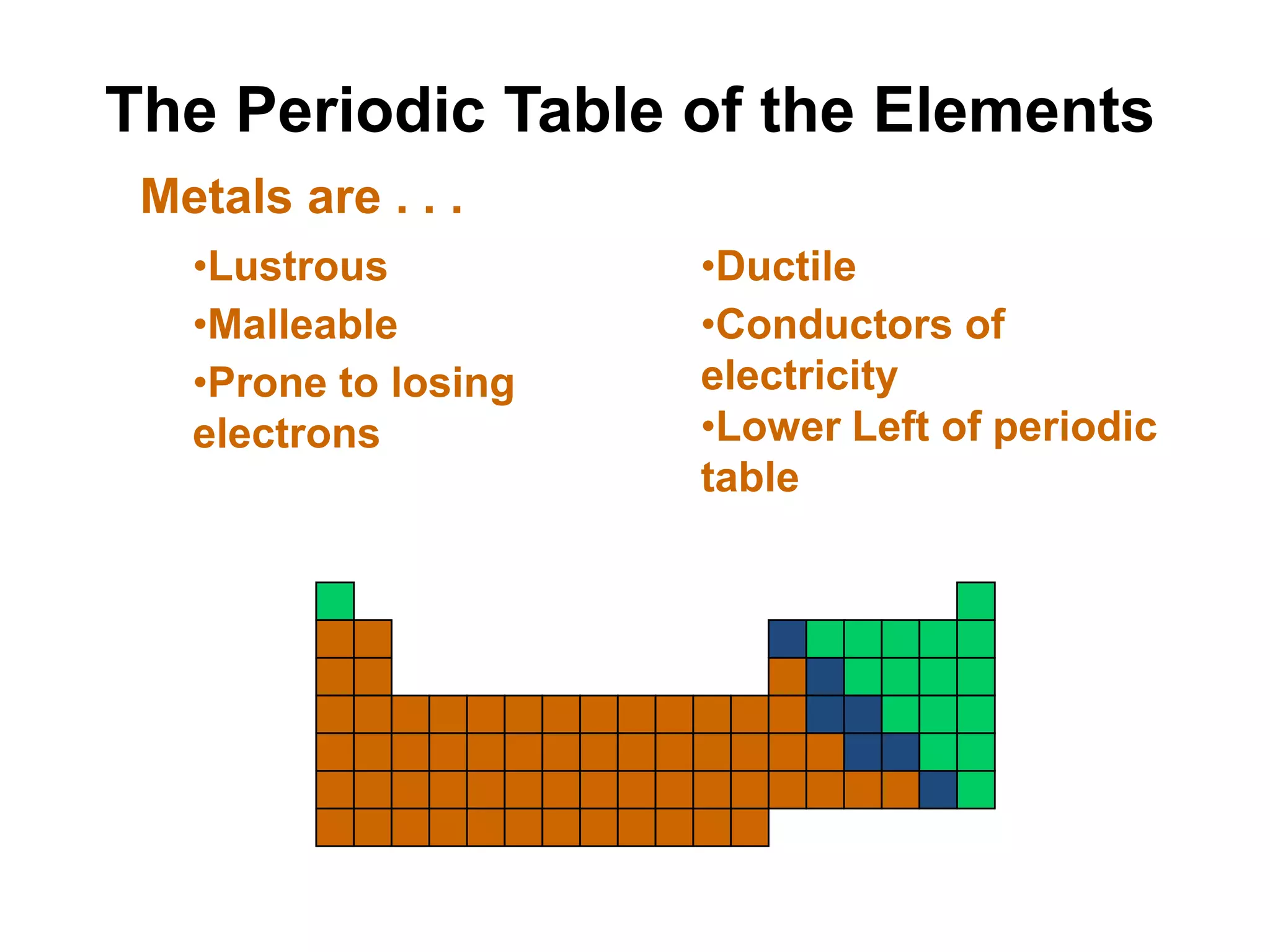
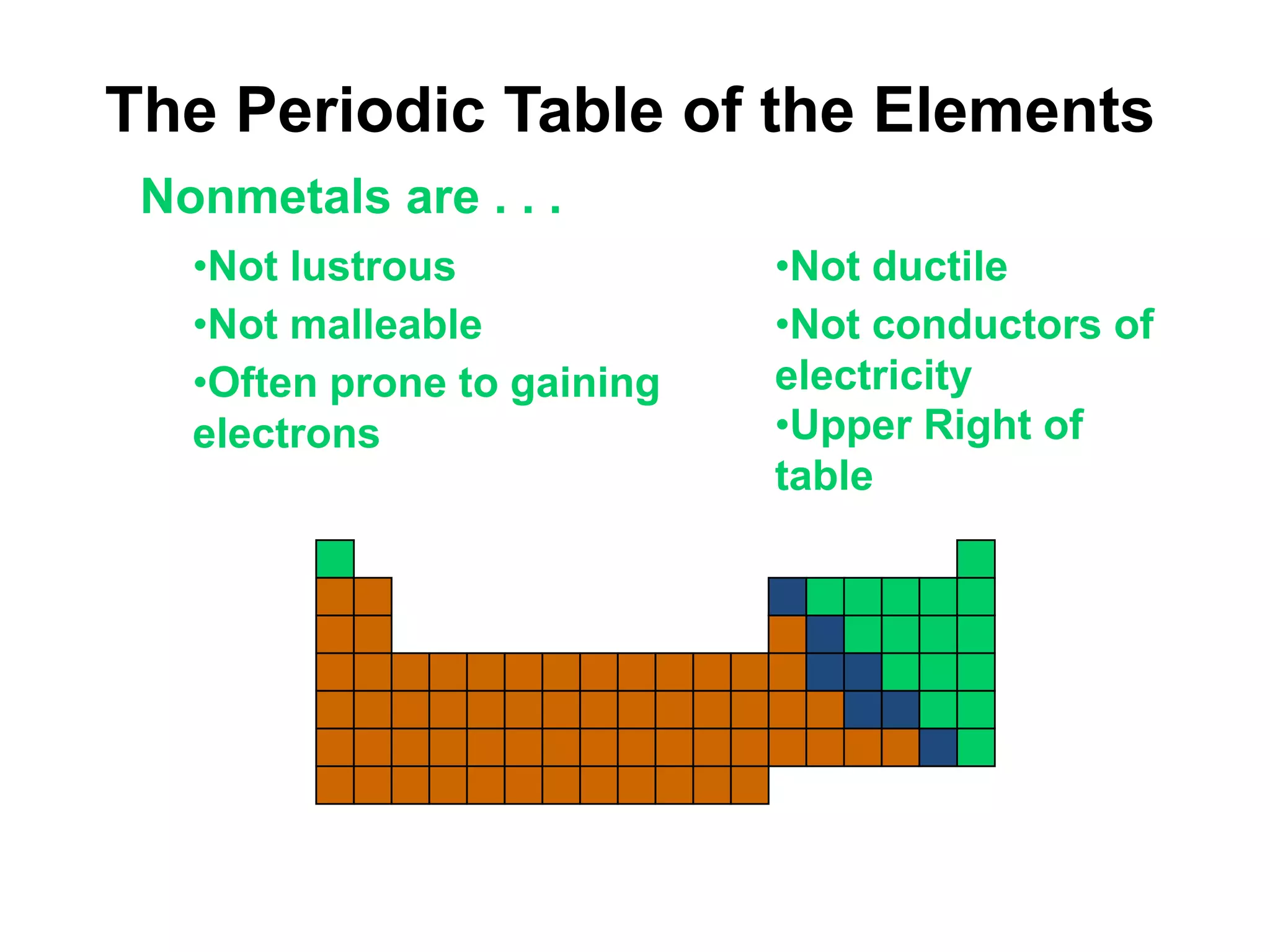
This document provides an overview of the syllabus and topics covered in a Chem 115: Basic Physiological Chemistry course taught by Dr. Kathryn Huisinga. The course will cover general chemistry, organic chemistry, and biochemistry topics as they relate to physiological processes. Students will learn about atoms, chemical structures and reactions, acids and bases, carbohydrates, lipids, proteins, DNA, metabolism and other biomolecules. The syllabus outlines expectations, assignments, grading policies, and the overall course schedule.