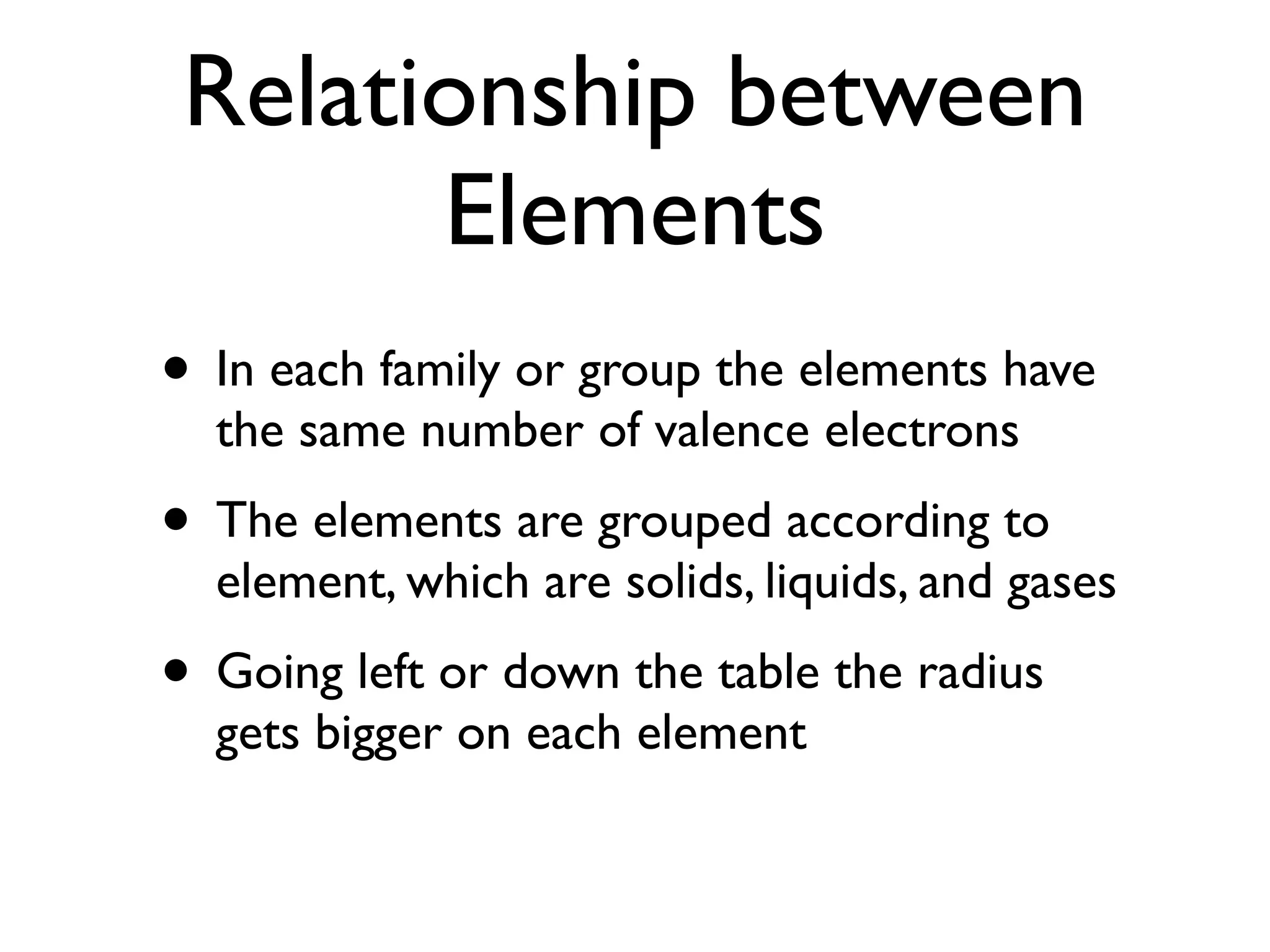
The Periodic Table organizes the chemical elements by atomic number. Dmitri Mendeleev is considered the inventor of the Periodic Table in 1869, arranging 63 known elements in order of atomic weight and properties. The table groups elements with similar properties together and orders them periodically by atomic number, metals, nonmetals, and metalloids. Elements in the same group have the same number of valence electrons.