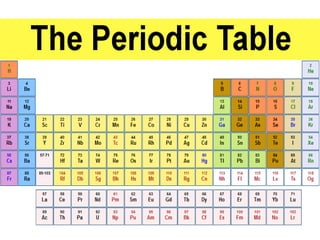
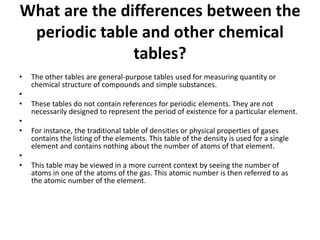
The document provides an overview of the periodic table, including its historical significance and the contributions of scientists like Mendeleev. It also discusses common misconceptions about the classification of elements and contrasts the periodic table with other chemical tables used for different purposes. The article aims to enhance understanding of chemistry and its complexities for students and enthusiasts.