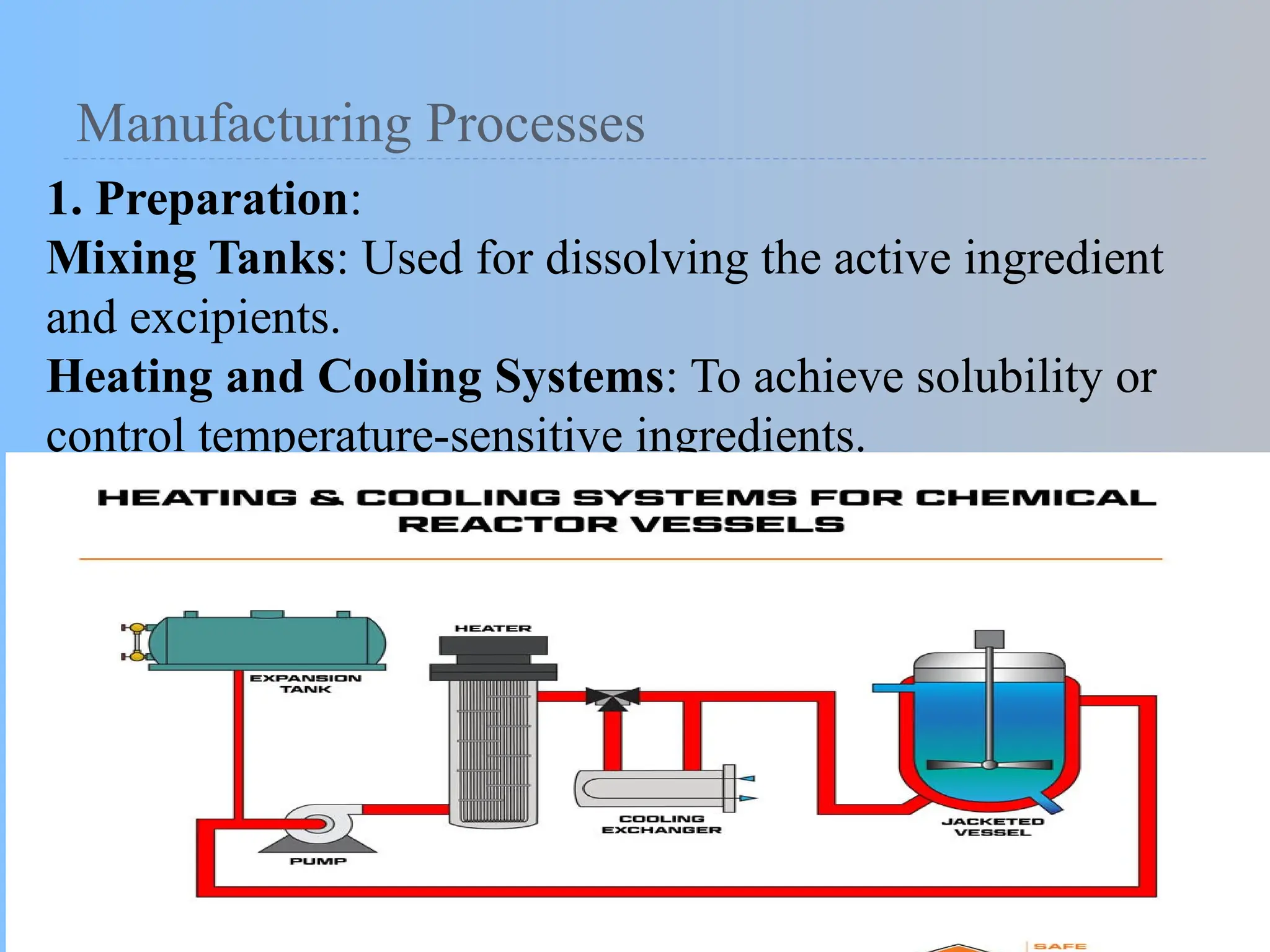
The document provides an overview of liquid dosage forms, focusing on solutions and dispersed systems, their formulation, advantages, disadvantages, and various excipients used in preparations. It covers critical aspects including methods to enhance solubility, manufacturing processes, and quality evaluations necessary for pharmaceutical liquids. Additionally, it lists the group members involved in the post-basic pharmacy project.