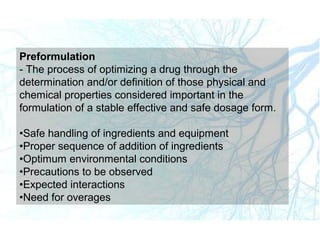
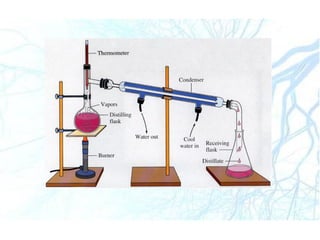
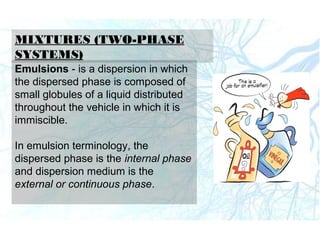
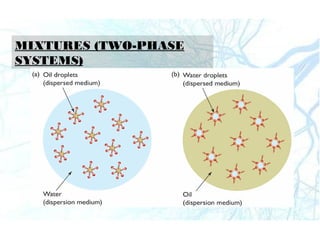
This document discusses liquid preparations including solutions, emulsions, and suspensions. It covers the key aspects of preformulation, types of solutions such as aqueous and non-aqueous, classification of emulsions, properties of suspensions, and methods of preparation for various liquid dosage forms like syrups, elixirs, and lotions. The focus is on optimizing drug delivery and stability through understanding physical and chemical properties and selecting appropriate ingredients and production techniques for different liquid preparations.