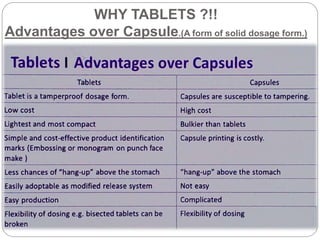
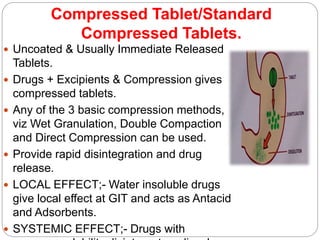
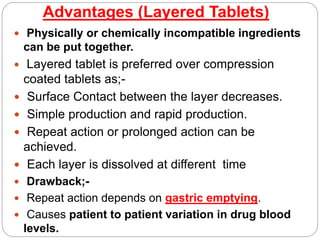
The document provides an overview of tablets, including their definition, ideal characteristics, classification, advantages, and disadvantages. Tablets are defined as compressed pharmaceutical solids and are the most common oral dosage form due to ease of administration. They are classified based on release mechanism (immediate vs sustained), administration route (oral, sublingual, etc.), and manufacturing method (compressed, molded, etc.). Compressed tablets have the highest production efficiency and lowest cost but may have bioavailability issues for some drugs. Other tablet types include effervescent, chewable, film coated, and controlled release tablets.