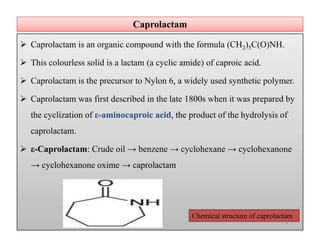
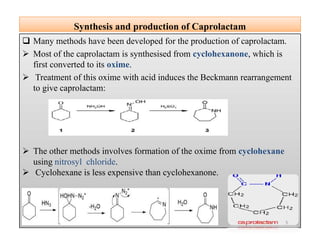
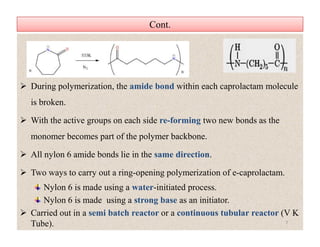
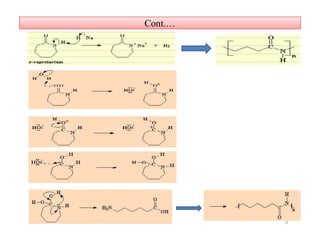
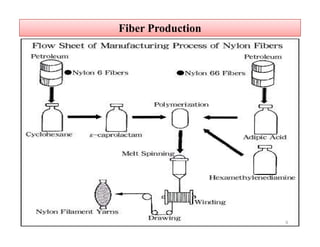
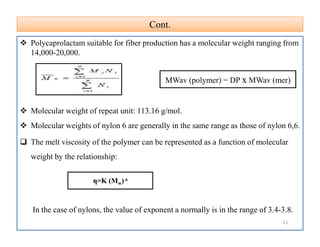
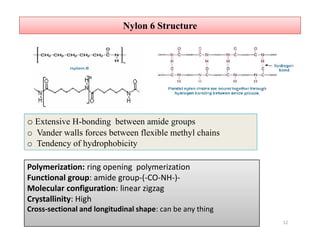
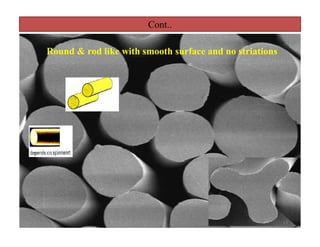
The document discusses nylon 6 fiber, including its production from caprolactam. Caprolactam is produced from cyclohexanone through several chemical reactions. Nylon 6 is synthesized through ring-opening polymerization of caprolactam. The polymerization occurs at high temperatures and results in a semi-crystalline structure. Nylon 6 fibers are produced through melt spinning and have properties derived from hydrogen bonding between amide groups and van der Waals forces between flexible chains.