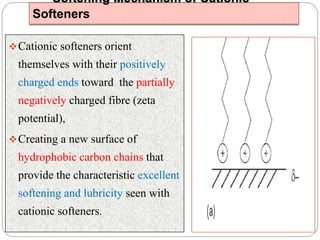
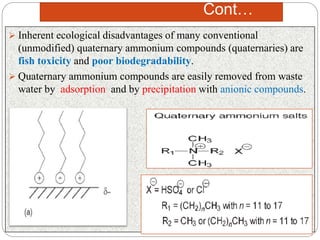
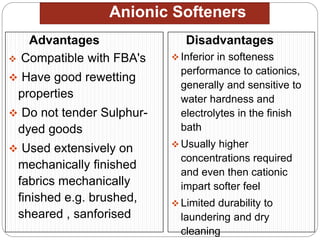
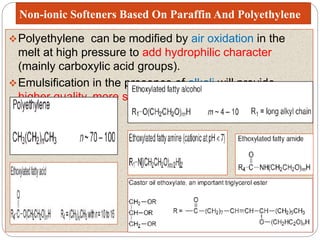
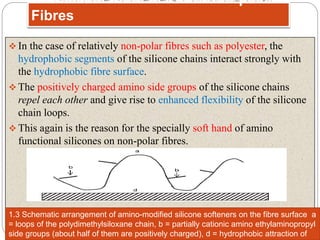
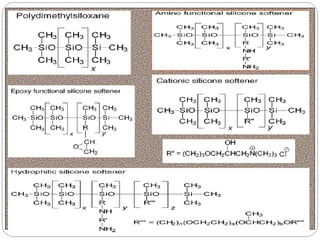
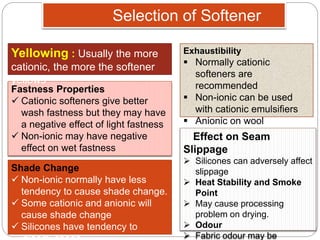
Softening finishes are important textile treatments that make fabrics feel softer. Chemical softeners allow fabrics to have a soft, smooth hand. The main types of softeners are cationic, anionic, non-ionic, and amphoteric softeners. Cationic softeners provide excellent softening but can cause yellowing, while anionic softeners have lower softening ability but better compatibility. Silicone softeners provide unique softness and properties like durability and heat stability, but can be expensive. Softener selection depends on the desired properties like fastness, compatibility with other chemicals, and effect on processes like seam slippage or drying.