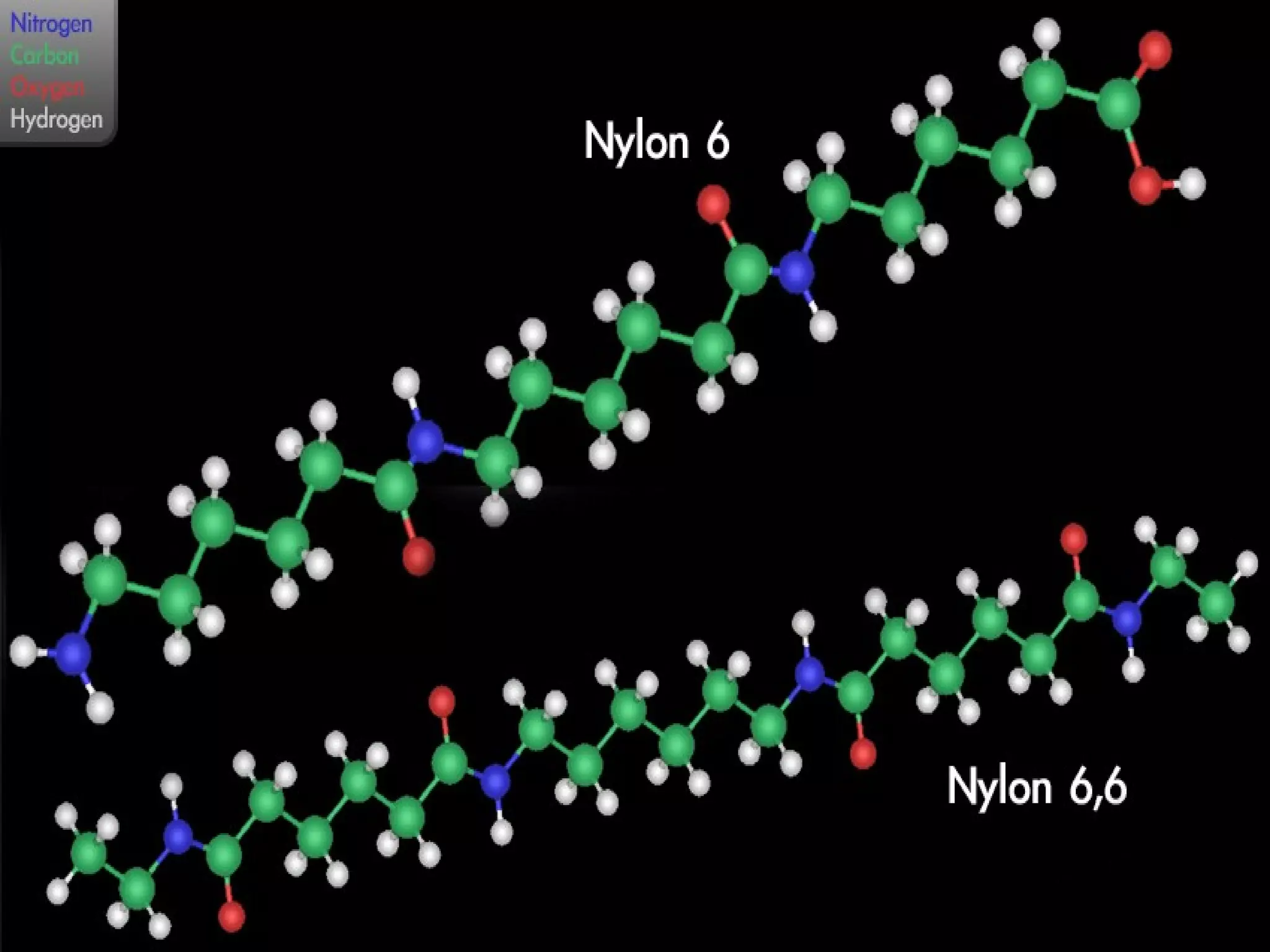
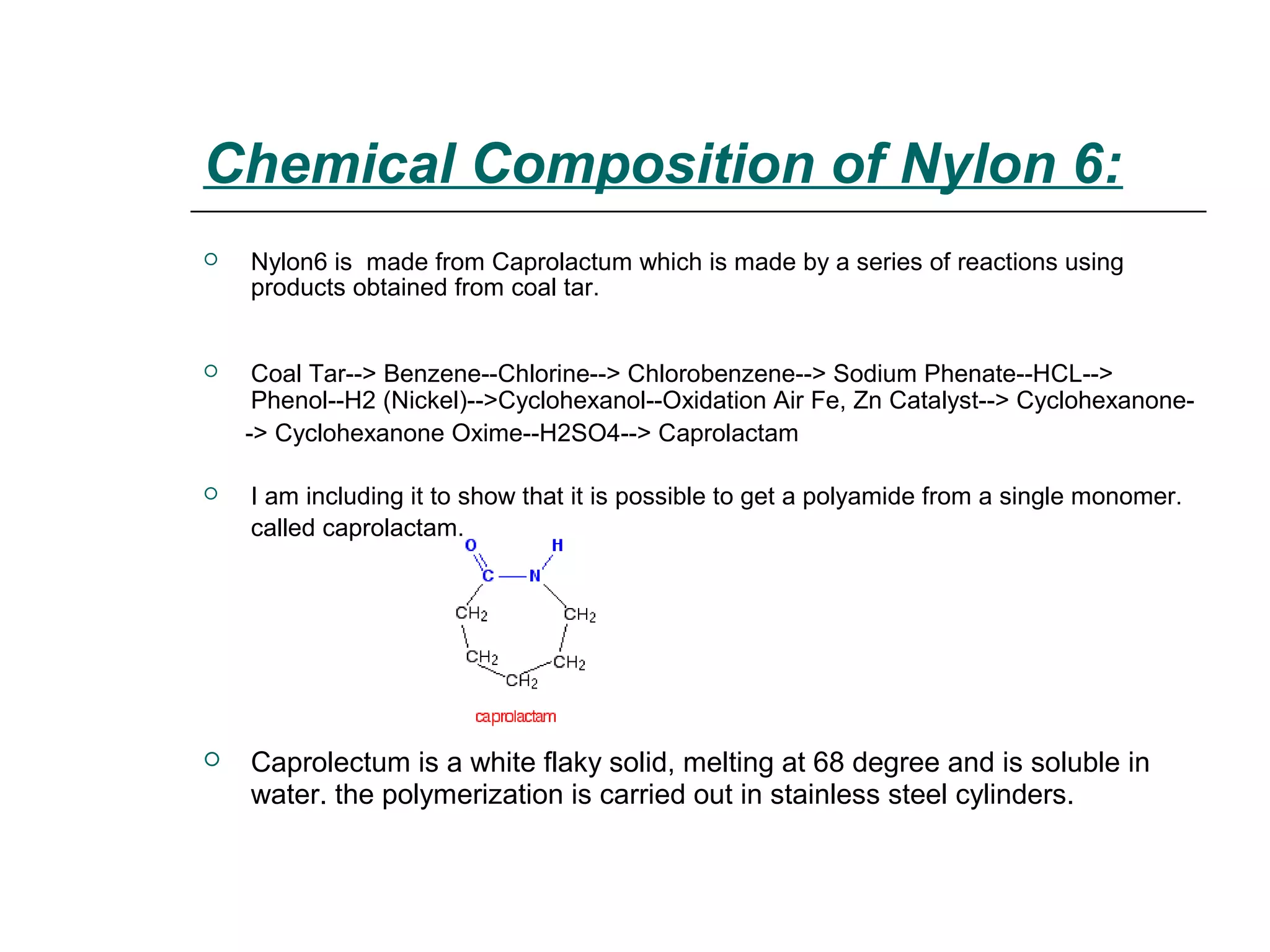
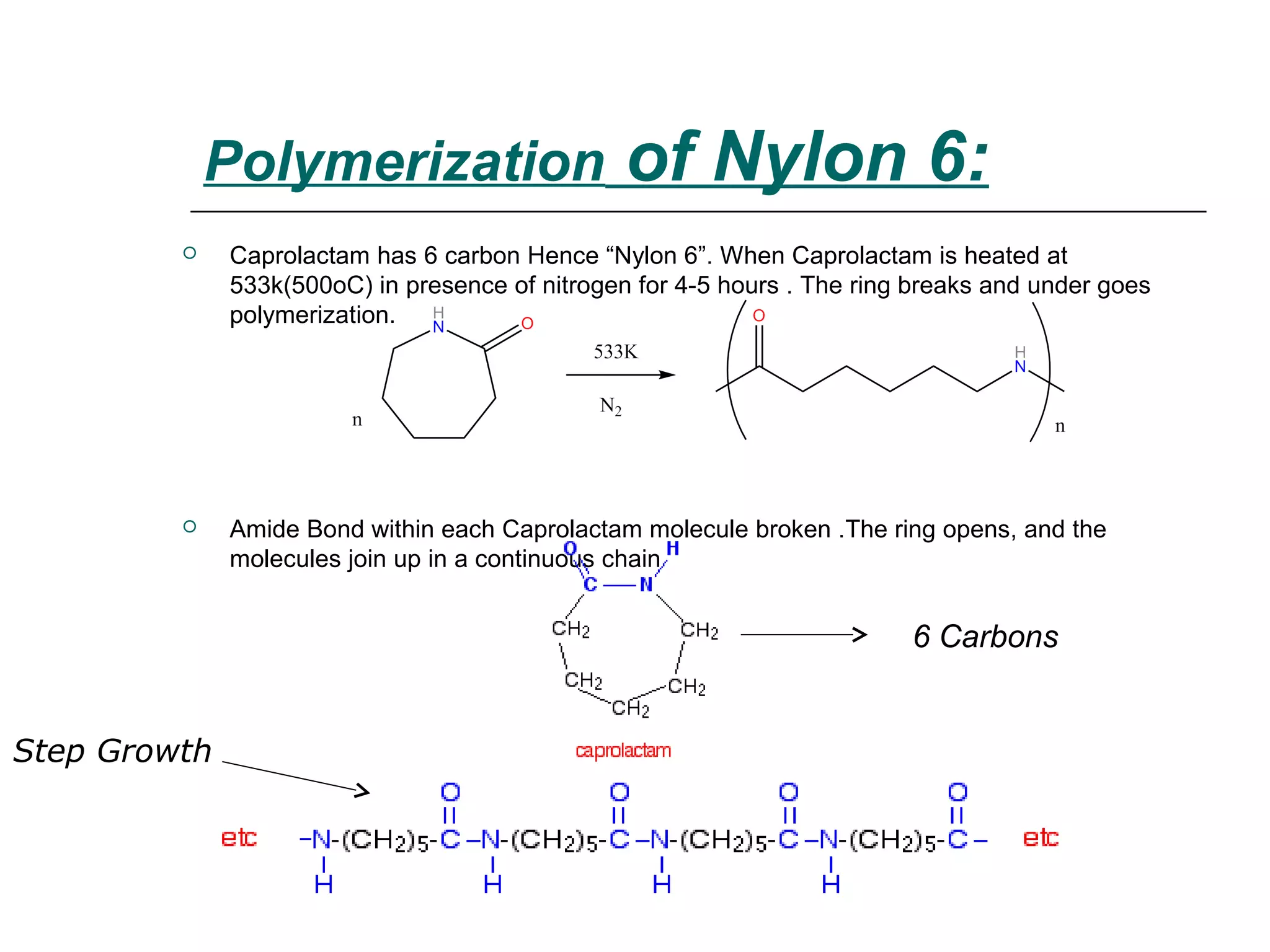
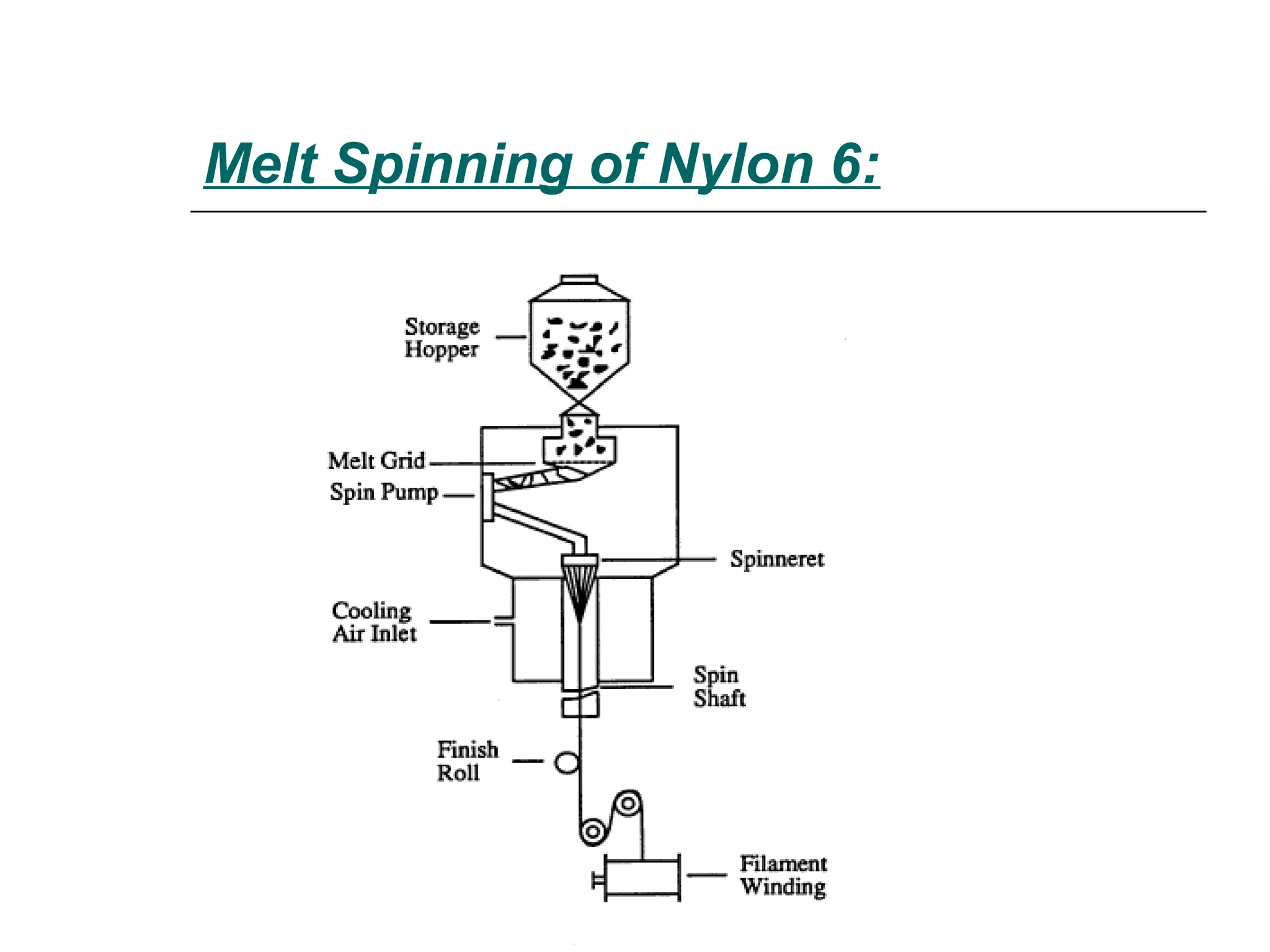
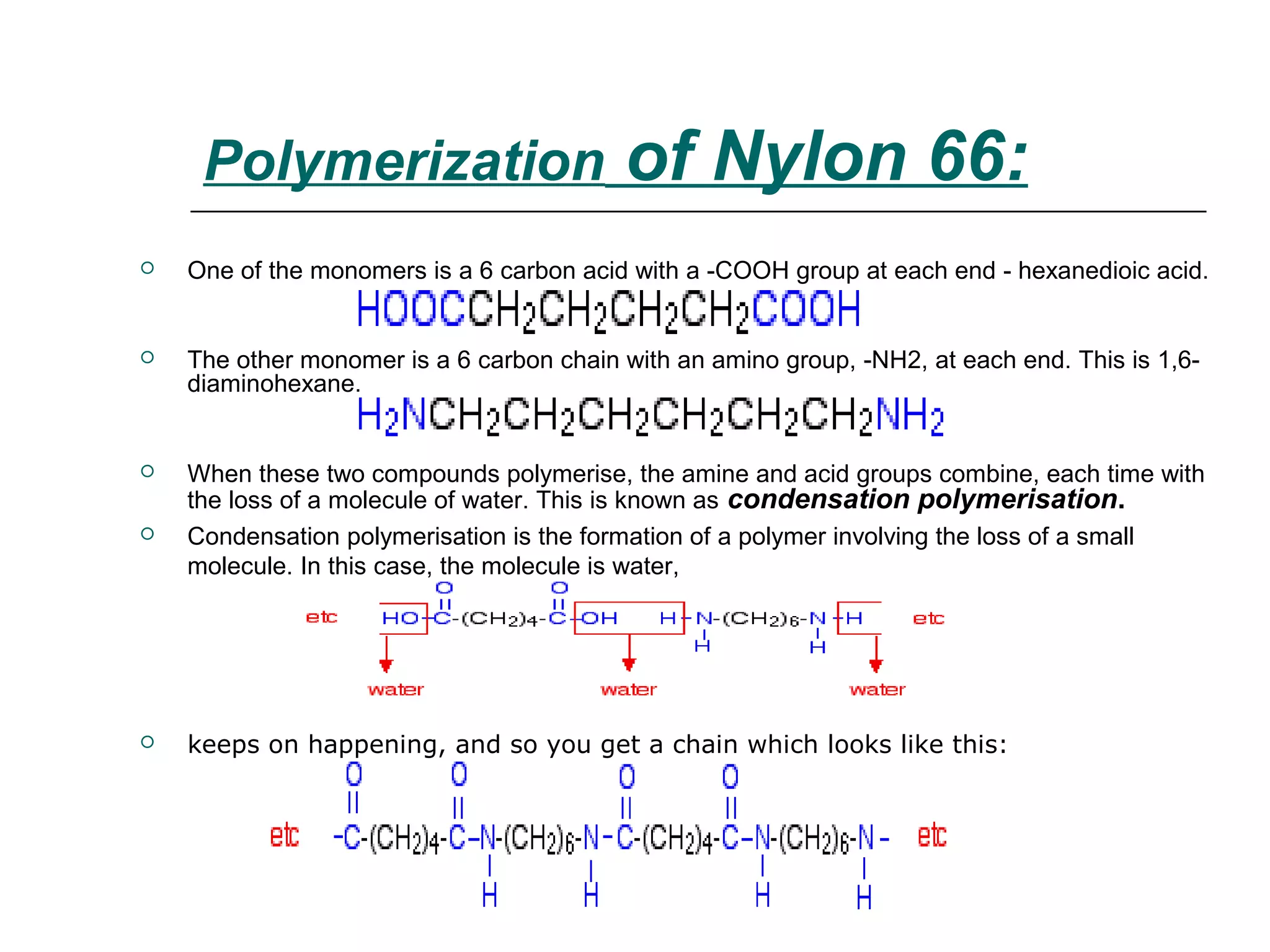
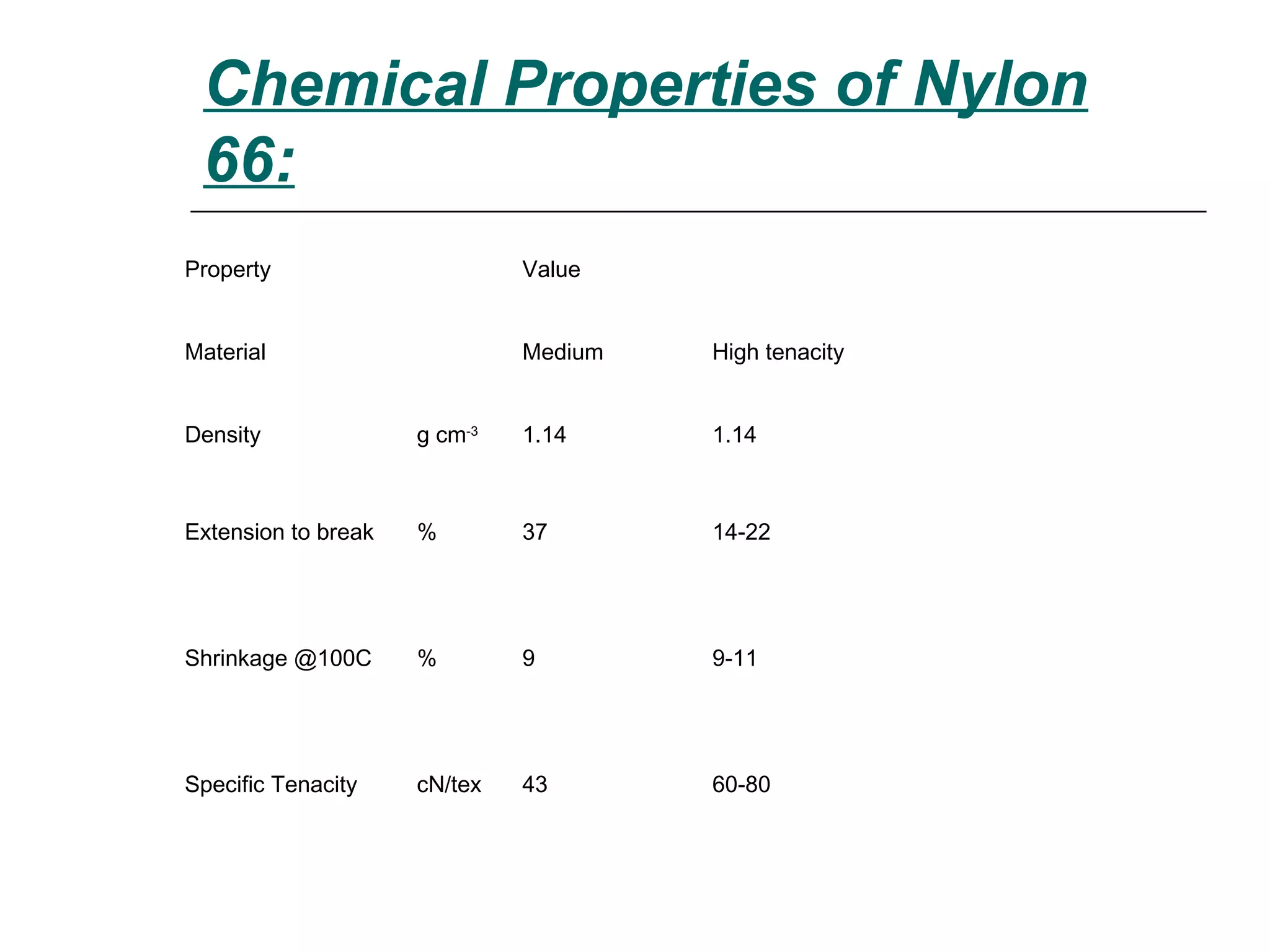
Polyamide fibers are composed of long molecular chains containing repeating amide groups. The two most common polyamides used to produce nylon fibers are nylon 6, which is made from caprolactam, and nylon 6,6, which is made from adipic acid and hexamethylenediamine. Nylon 6 is produced by polymerizing caprolactam, a white solid made from coal tar derivatives, above 500°C. Nylon 6,6 is formed through condensation polymerization of adipic acid and hexamethylenediamine with the loss of water. Both nylons are melt spun into fibers and widely used in applications such as fabrics, carpets, ropes and tires