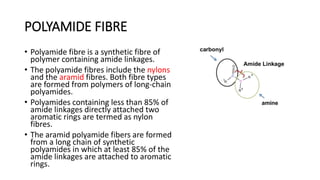
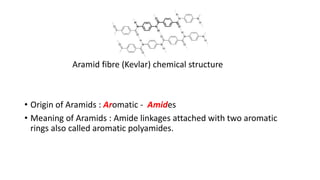
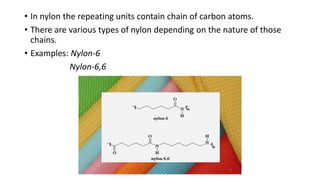
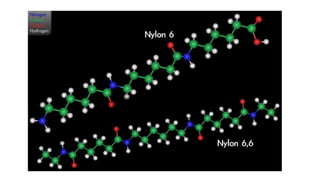
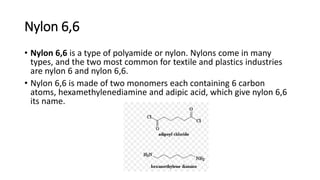
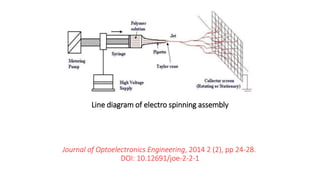
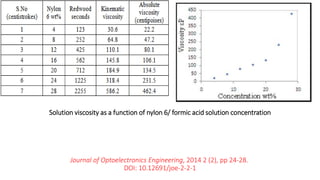
This document discusses nylon fibers, including their synthesis, properties, and applications, highlighting types like nylon 6 and nylon 6,6. It describes the characteristics of polyamide fibers, including their strength, durability, and resistance to chemicals, as well as their use in various industries such as textiles and automotive. Additionally, it covers the preparation of nylon fibers using electrospinning techniques and their modern applications in clothing, sports equipment, and medical devices.