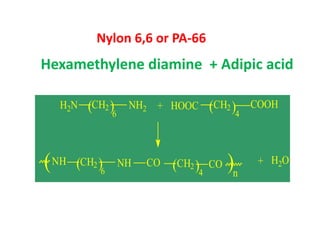
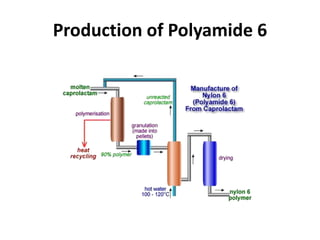
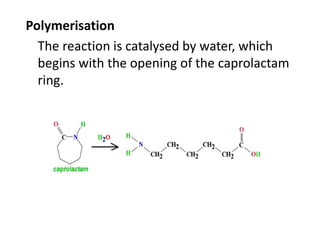
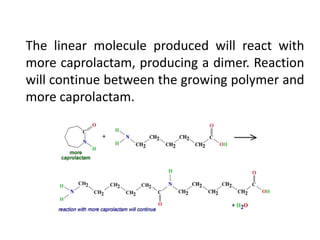
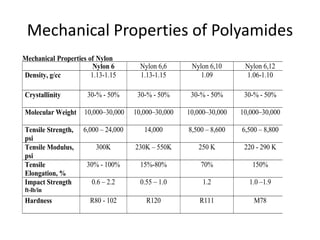
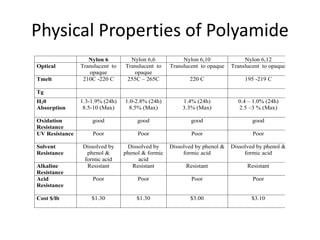
Polyamides, also known as nylons, are polymers containing amide bonds along the polymer chain. Naturally occurring polyamides include proteins like wool and silk, while synthetic polyamides like nylon 6 and nylon 6,6 are produced through polymerization reactions and are widely used in textiles, automotive parts, and other applications due to their strength and durability. Polyamides are synthesized from monomers like caprolactam and can be processed via common plastic molding techniques. They possess good mechanical properties but also have limitations such as moisture absorption.