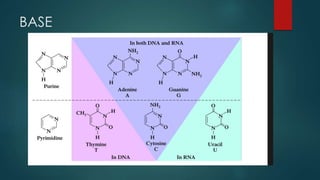
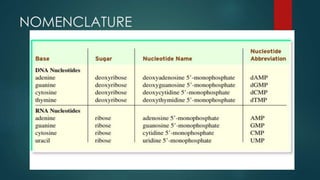
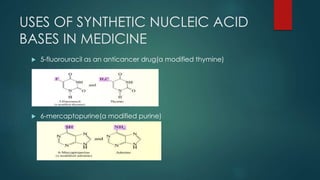
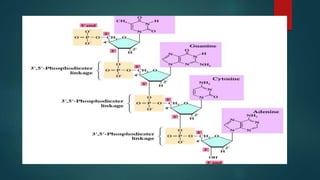
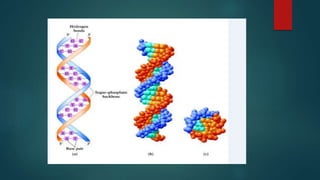
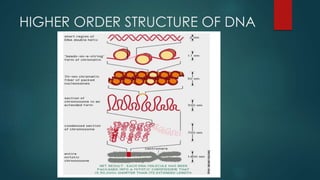
1) Nucleic acids are biopolymers made of nucleotides that contain three components - a nitrogenous base, a 5-carbon sugar, and a phosphate group. 2) DNA contains the bases adenine, guanine, cytosine and thymine, while RNA contains adenine, guanine, cytosine and uracil instead of thymine. 3) Both DNA and RNA are polymers of nucleotides, and DNA typically takes the form of a double helix due to base pairing between adenine and thymine and between guanine and cytosine.