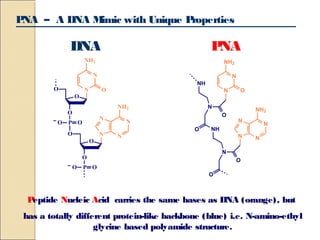
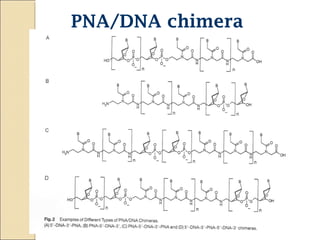
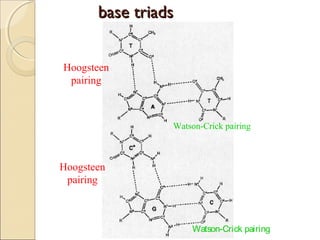
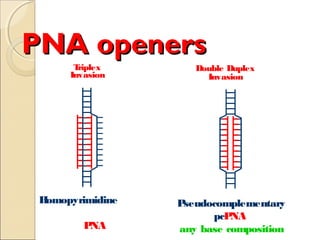
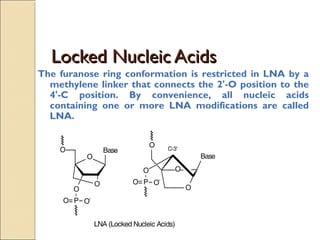
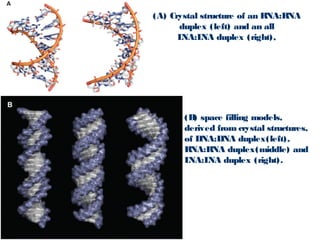
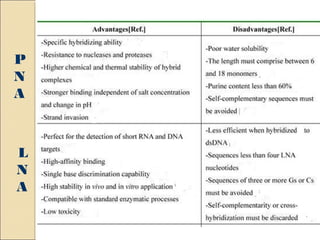
This document discusses two types of nucleic acid mimetics called PNA and LNA. PNA uses a peptide-like backbone instead of a sugar-phosphate backbone, allowing it to bind strongly to DNA and RNA. LNA contains a methylene bridge that locks the furanose ring into a rigid conformation, increasing binding affinity and specificity. Both PNA and LNA can be used for applications like antisense therapy, gene regulation, and molecular diagnostics due to their high binding affinity and specificity for complementary nucleic acid sequences.