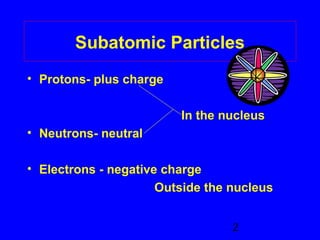
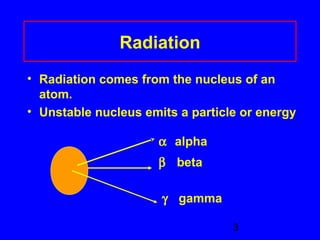
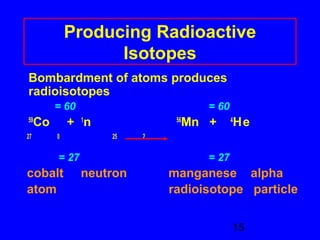
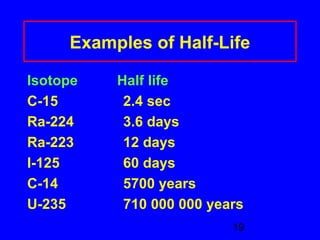
This document provides information about nuclear radiation and nuclear reactions. It defines different types of radiation such as alpha, beta, and gamma radiation. It explains nuclear equations and how the number of protons and neutrons must balance. It discusses radioactive decay and half-life. It also describes the differences between nuclear fission, where large nuclei split into smaller pieces, and nuclear fusion, where small nuclei combine to form larger nuclei. Fission occurs in nuclear power plants and bombs while fusion powers the sun.