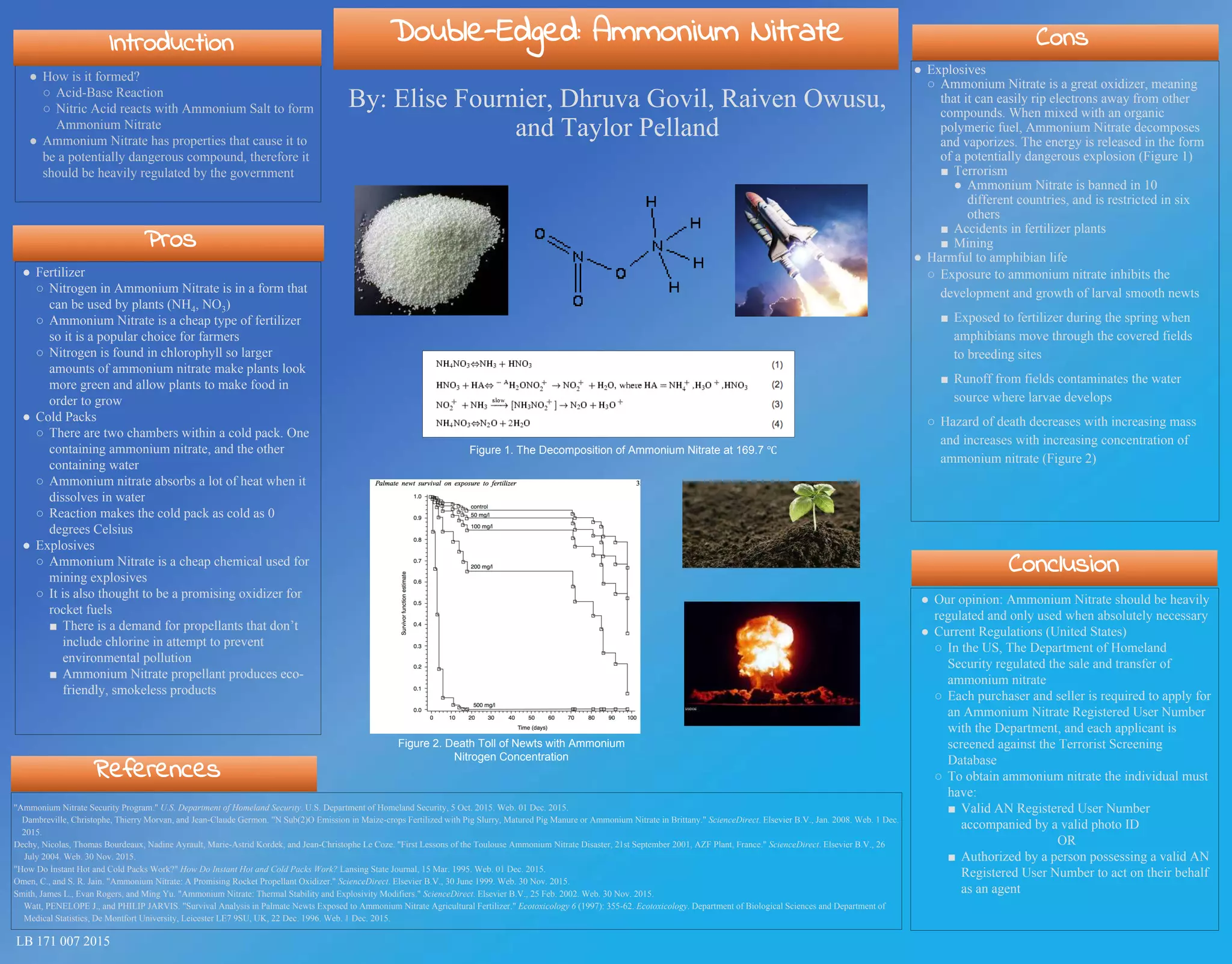
Ammonium nitrate is a double-edged compound that can be used as a fertilizer but also has dangerous explosive properties. It is used as a cheap fertilizer for farms but can inhibit the growth of amphibian larvae if runoff contaminates water sources. Ammonium nitrate is also used in mining explosives and is a promising rocket fuel oxidizer, but it has been used in acts of terrorism and caused industrial accidents, so many countries heavily regulate its sale and use.