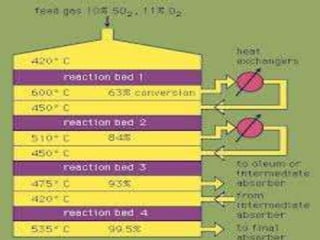
The document describes the contact process for producing sulfuric acid from sulfur. It involves burning sulfur to produce sulfur dioxide, then converting the sulfur dioxide to sulfur trioxide in a converter using a catalyst and air. The sulfur trioxide is then absorbed in concentrated sulfuric acid in an absorption tower to produce more sulfuric acid.