Embed presentation
Downloaded 23 times


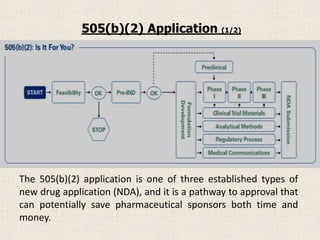




The document discusses the three types of New Drug Applications (NDAs) used in the United States to seek FDA approval for new pharmaceutical products. A "full" NDA contains clinical trial data proving safety and efficacy. A 505(b)(2) application relies partly on published information and can potentially save time and money compared to a full application. It is best suited for products representing limited changes to previously approved drugs, like new formulations or indications. An abbreviated application under 505(j) is for generics proving only bioequivalence to an existing drug.





