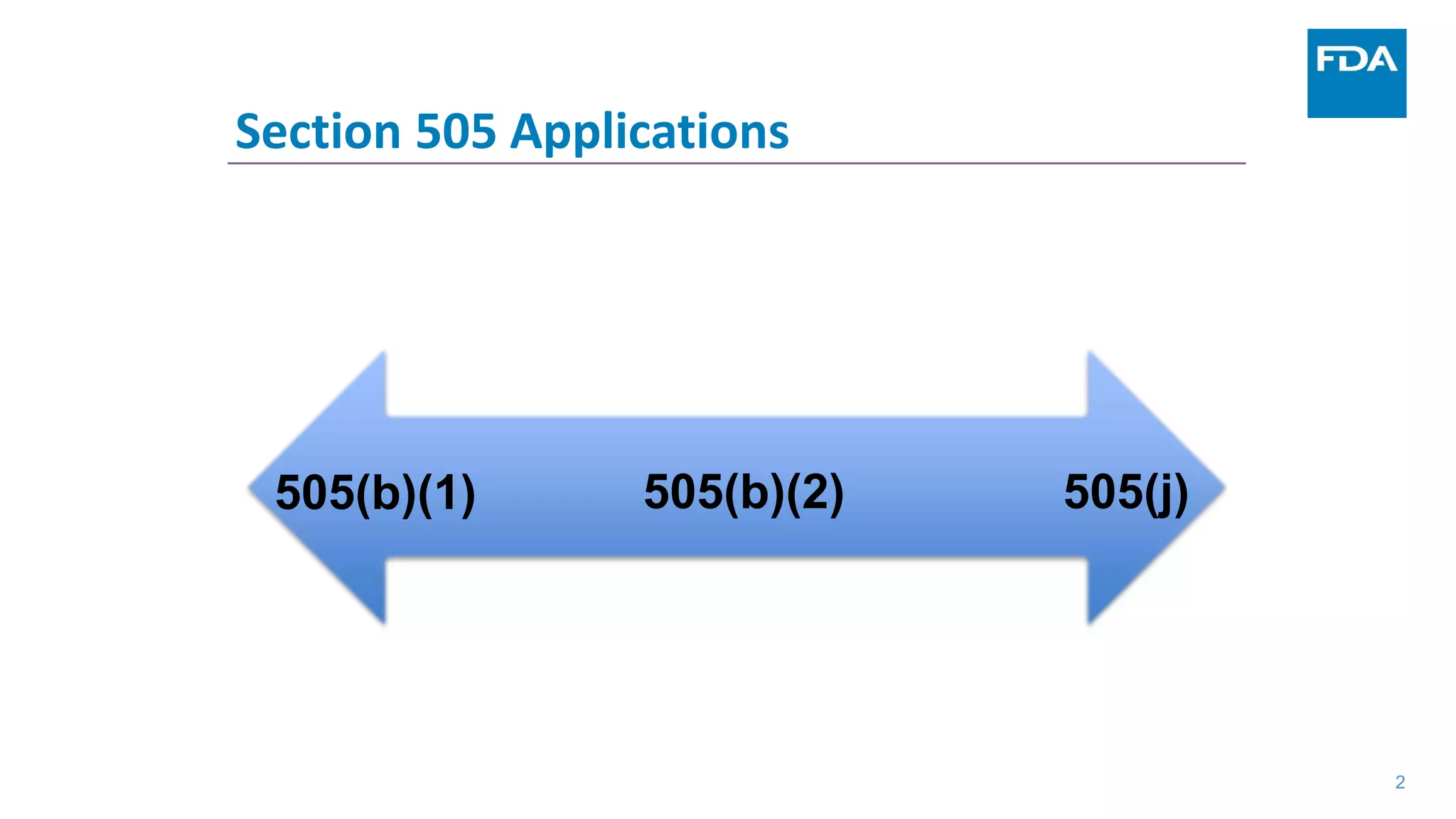
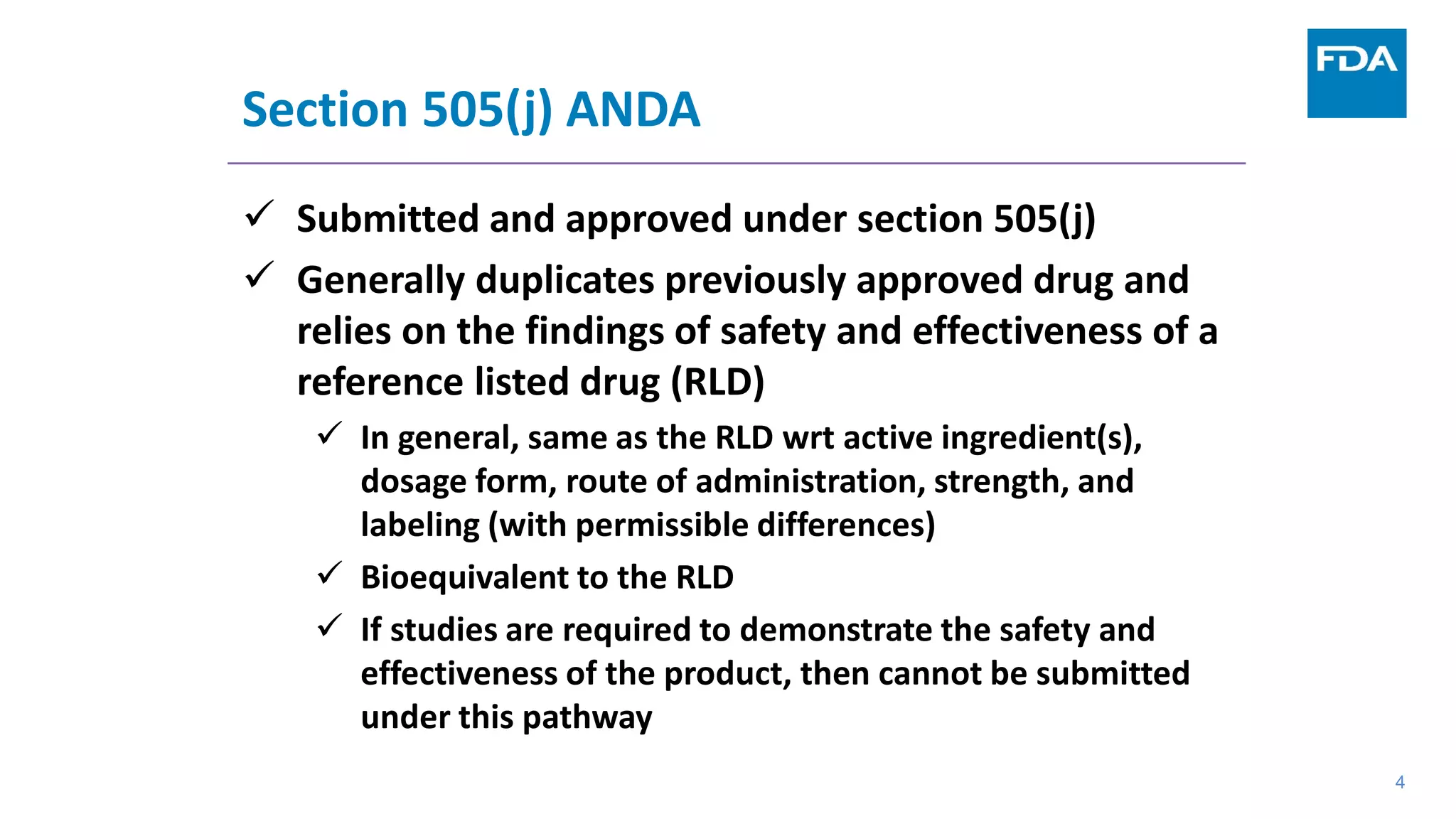
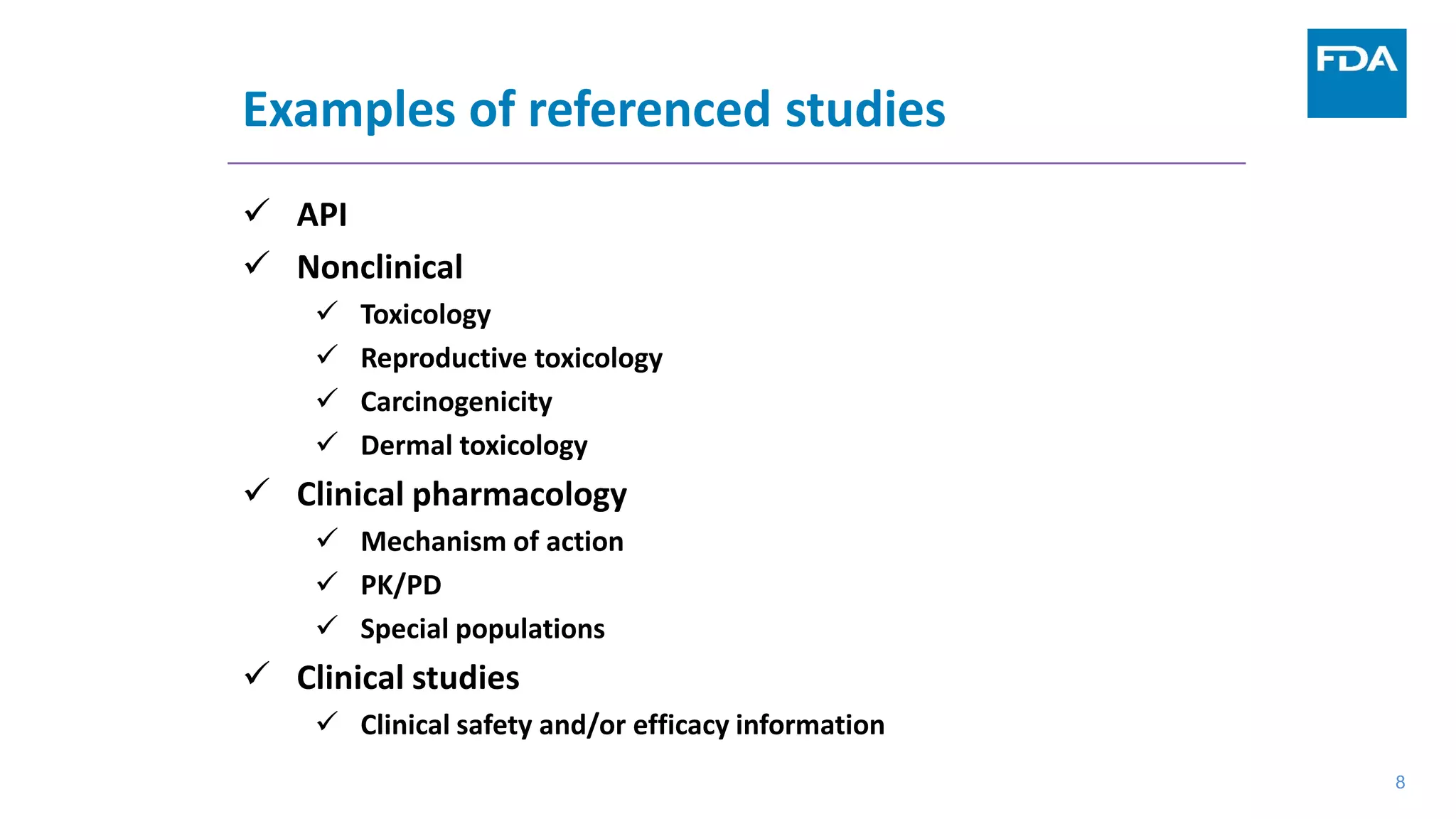
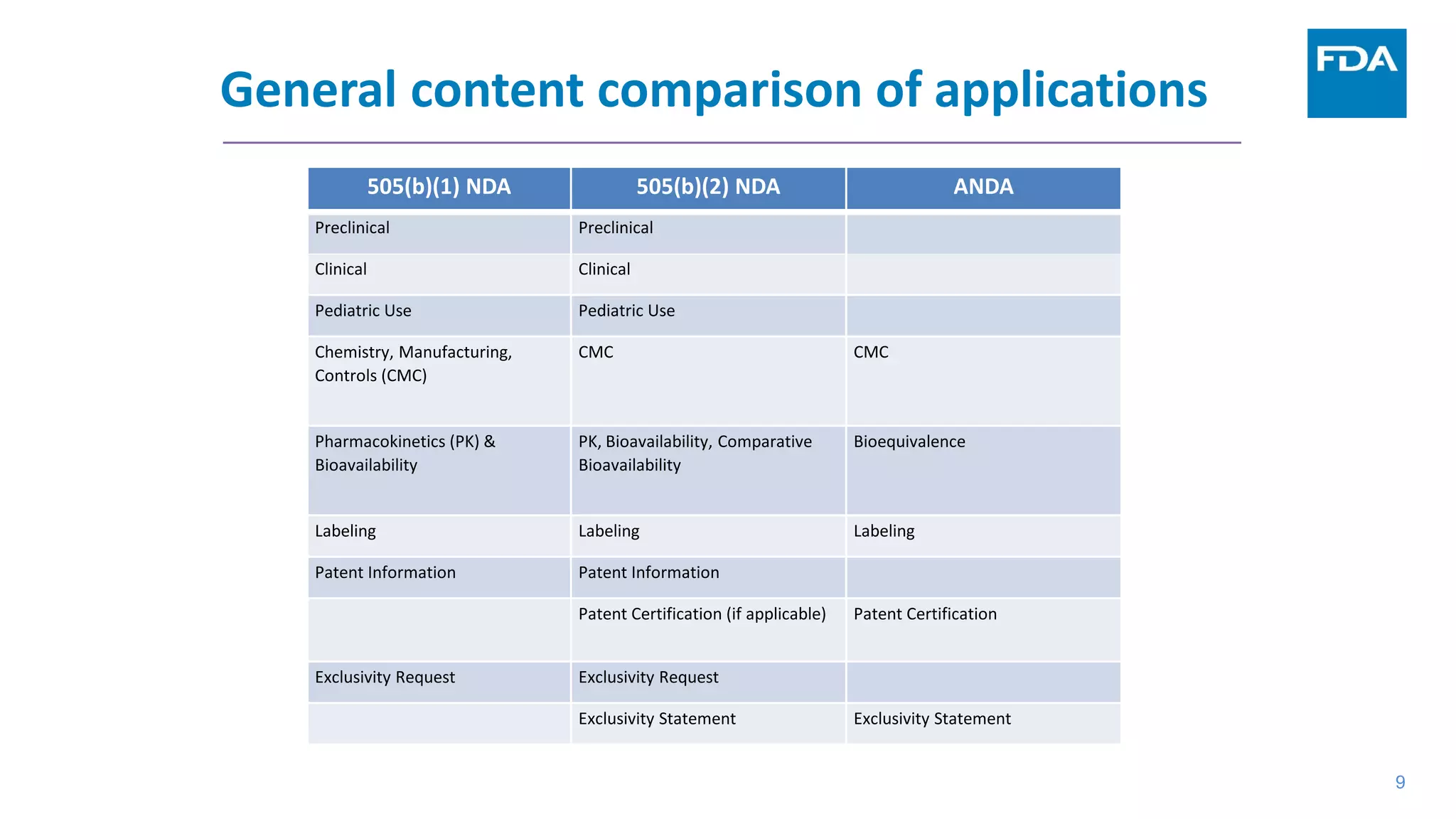
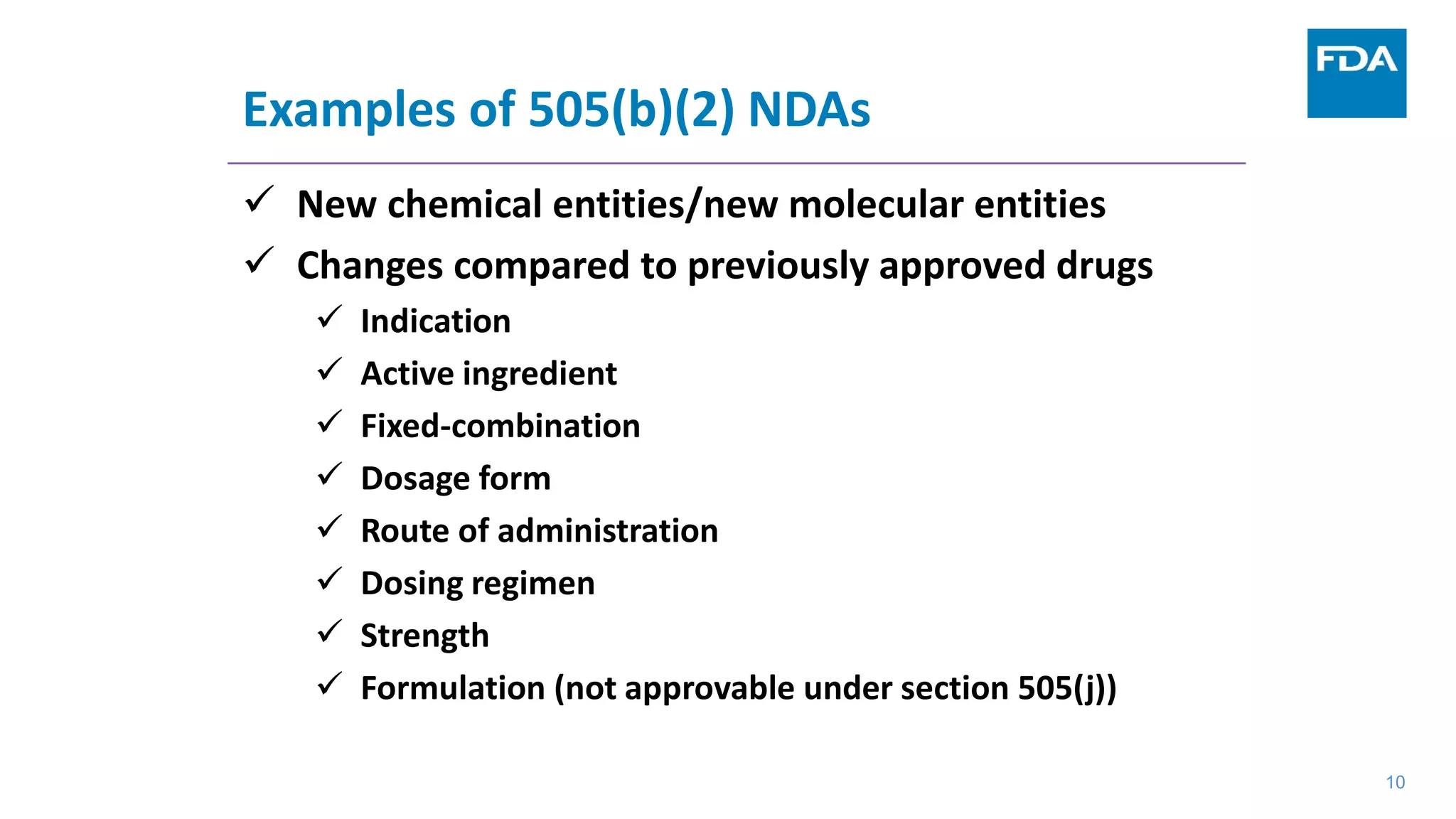
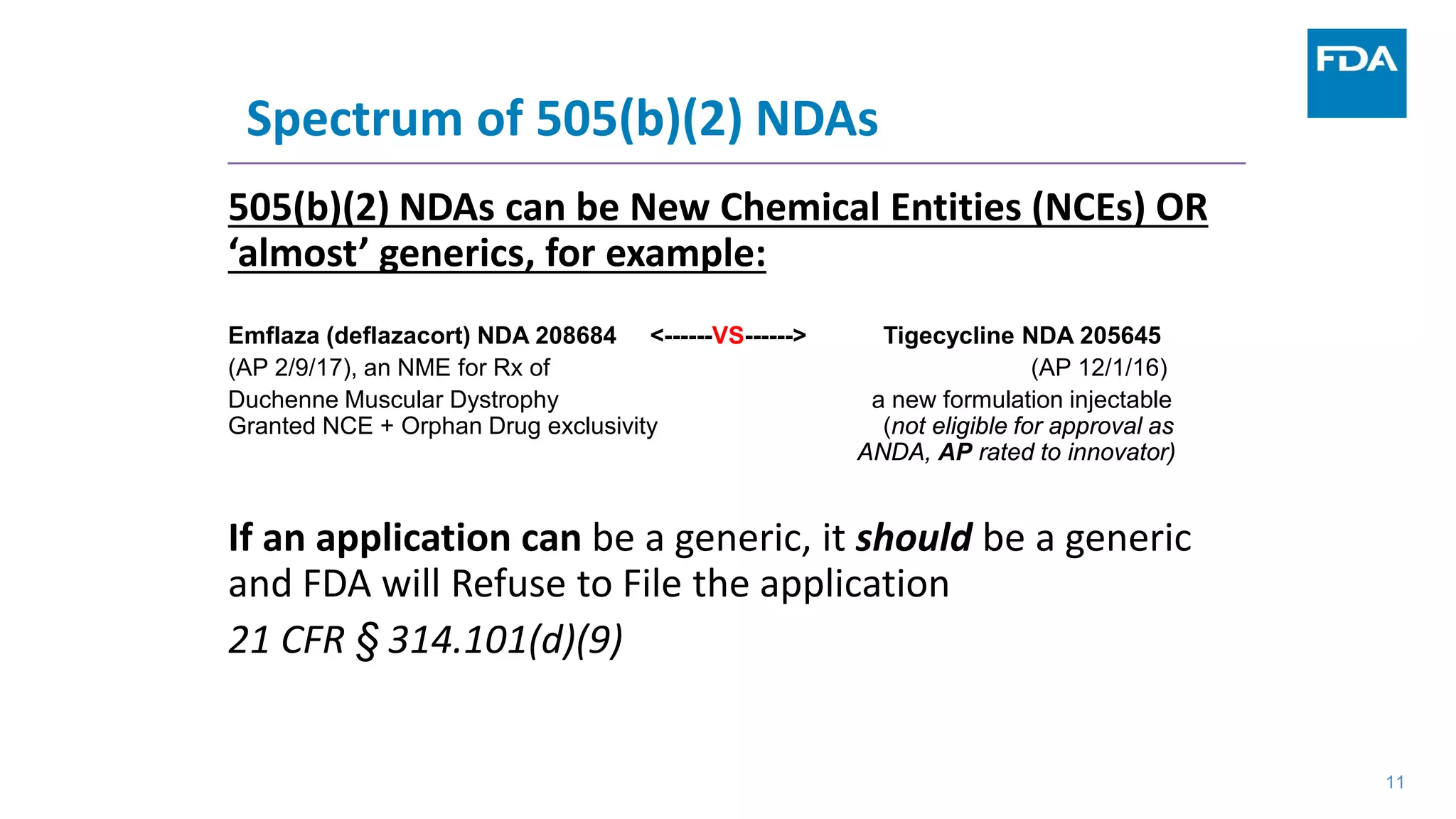
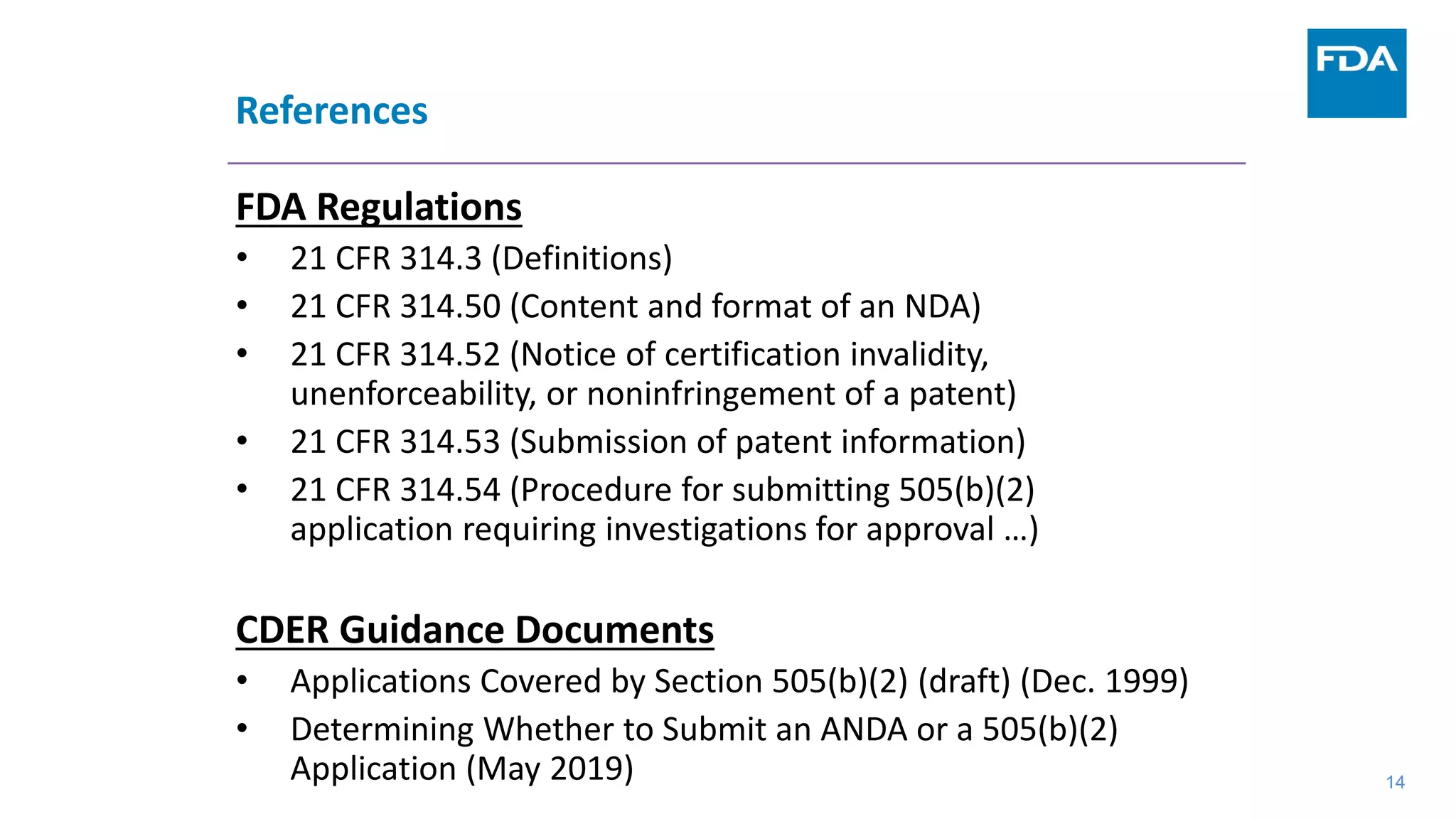
This document provides an overview of the 505(b)(2) regulatory pathway for new drug applications at the FDA. It describes how 505(b)(2) NDAs can rely on previously approved drugs for certain required information, allowing flexibility without having to conduct all new clinical studies. Examples are given of types of drugs that may be submitted via the 505(b)(2) pathway as well as potential sources of reliance such as published literature, FDA findings, or bridging studies to an approved drug. Requirements for patents and potential market exclusivity are also summarized.