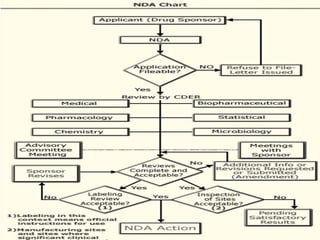
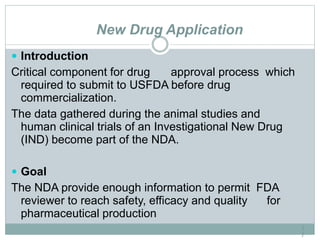
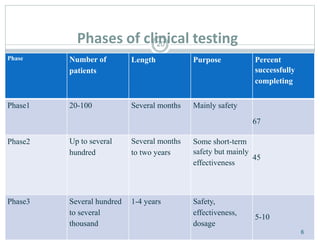
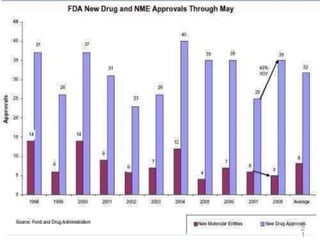
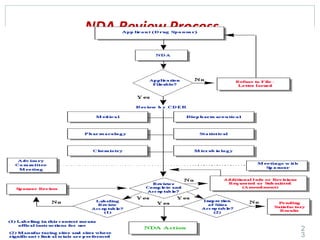
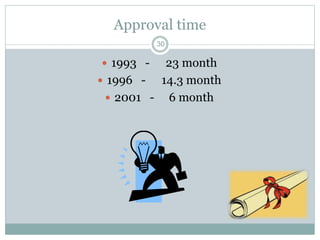
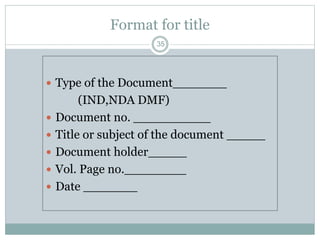
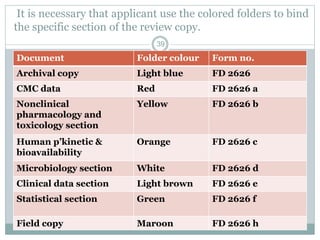
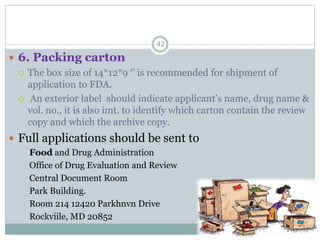
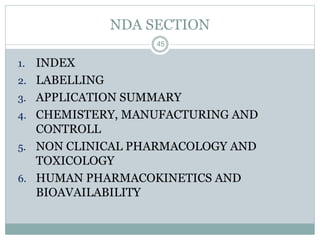
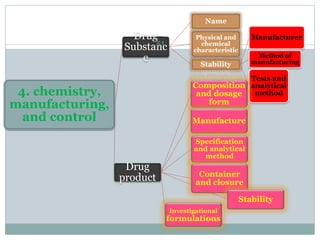
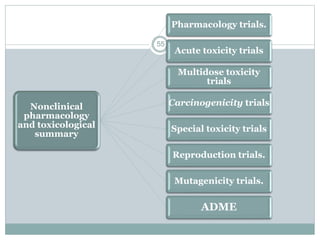
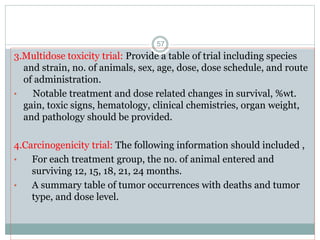
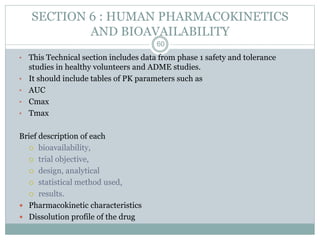
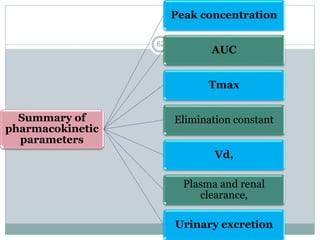
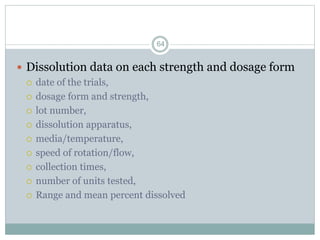
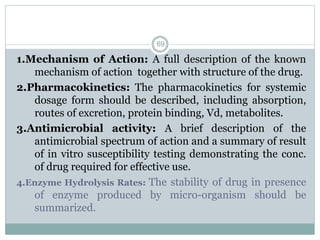
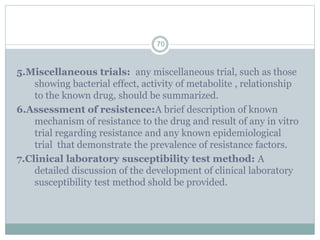
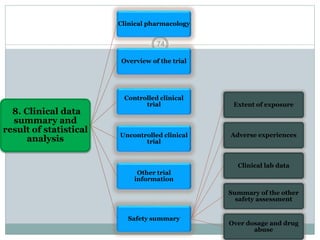
The document provides information about the New Drug Application (NDA) process. The NDA is submitted to the FDA to obtain approval for a new pharmaceutical. It contains clinical and non-clinical data, chemistry information, and manufacturing details. The NDA follows guidelines for formatting and submission. It includes an archival copy that contains all sections and a review copy divided into technical sections bound with colored covers. The document outlines the contents and organization required for each section of the NDA.