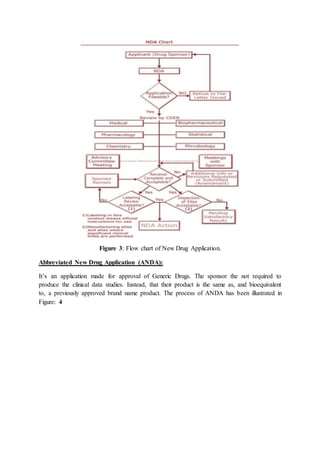
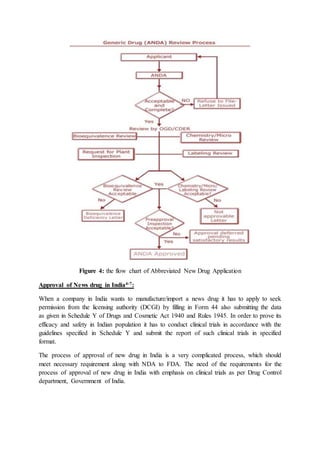
The document compares the drug approval processes in the US and India. In the US, the process involves an Investigational New Drug (IND) application to begin clinical trials, followed by a New Drug Application (NDA) if clinical studies show the drug is safe and effective. In India, companies must apply for permission by submitting data to the Drugs Controller General of India and conduct clinical trials according to guidelines. Both countries have stringent approval standards aimed at safeguarding public health by ensuring drugs are properly tested and manufactured.