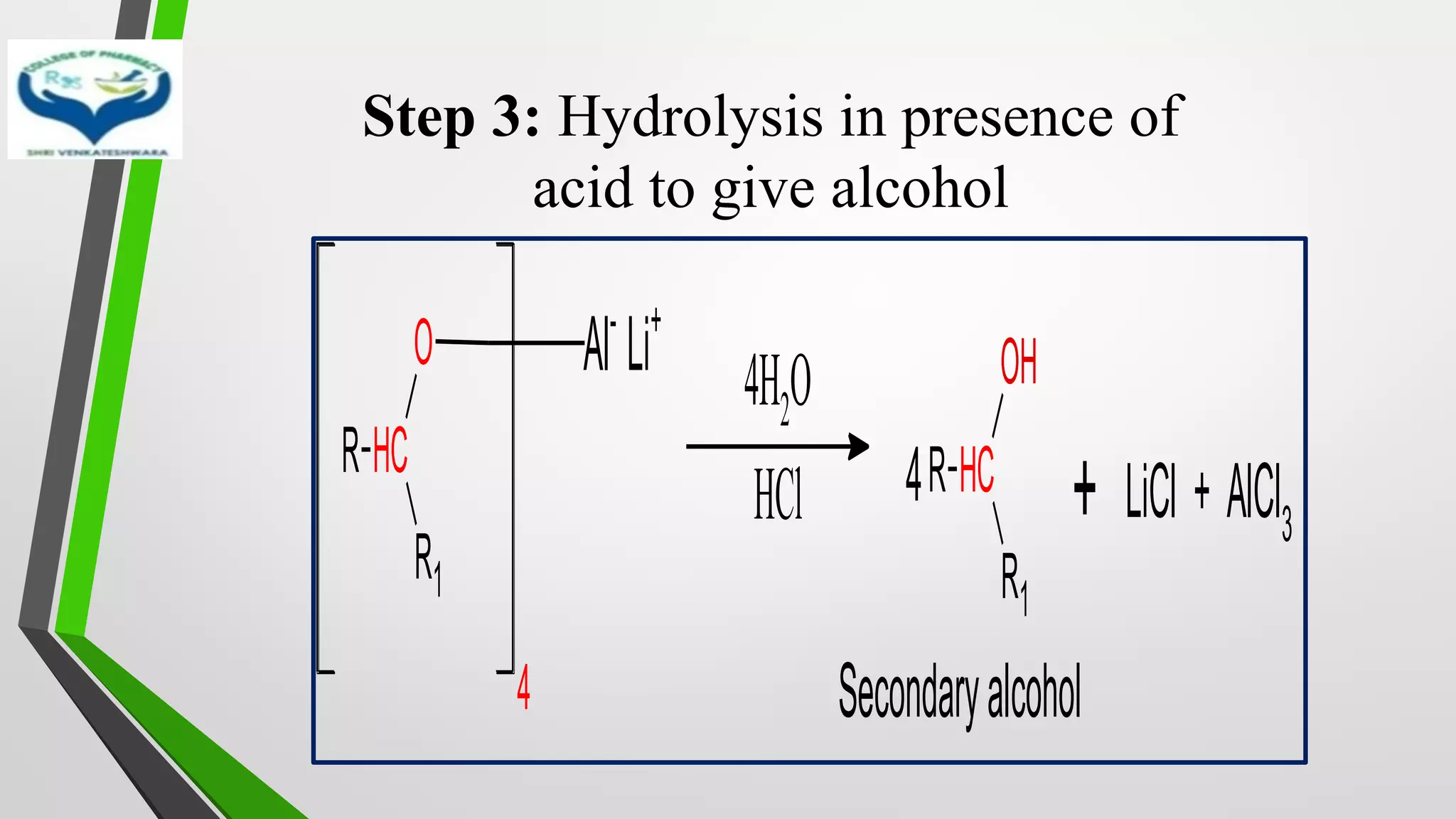
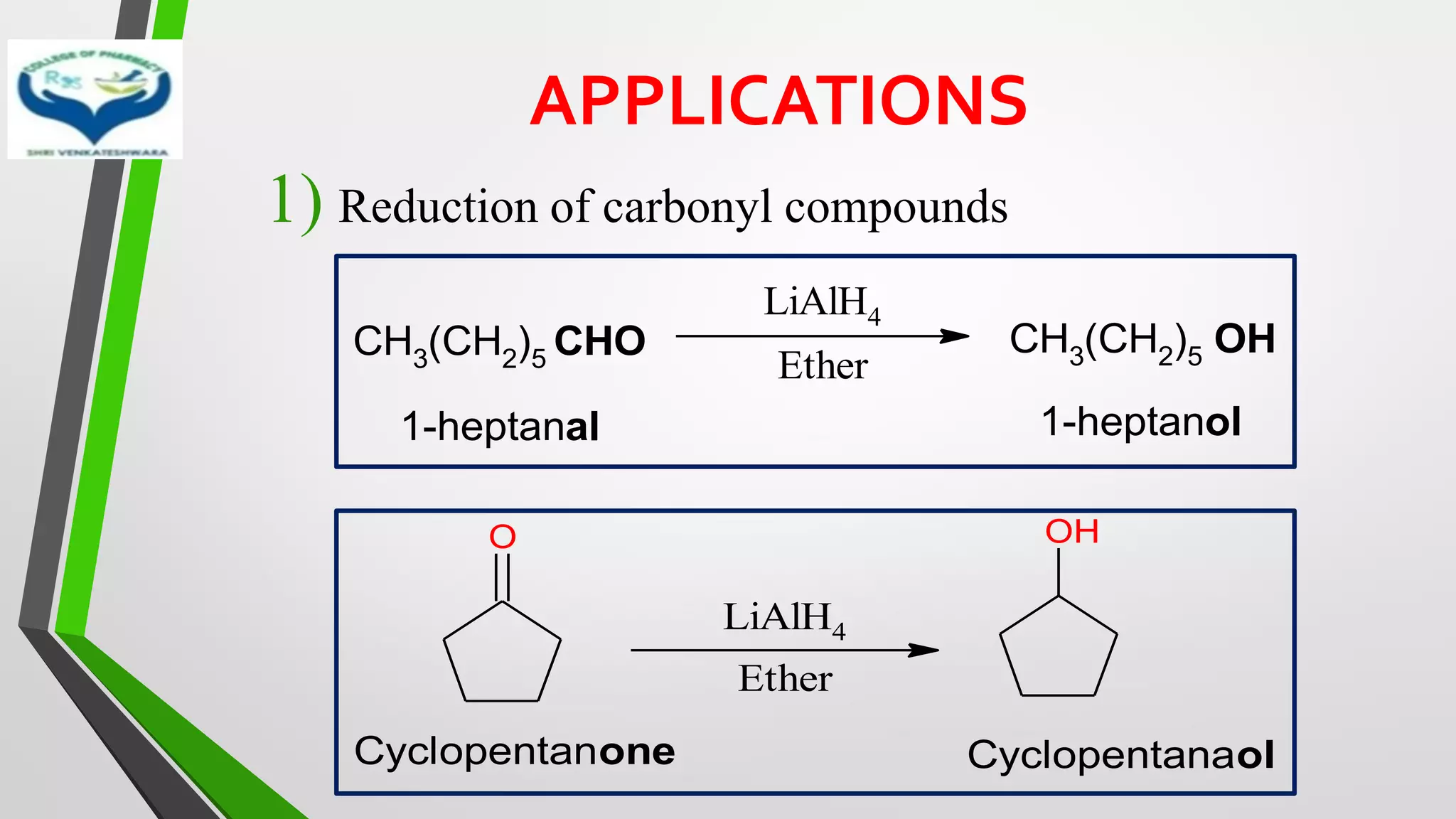
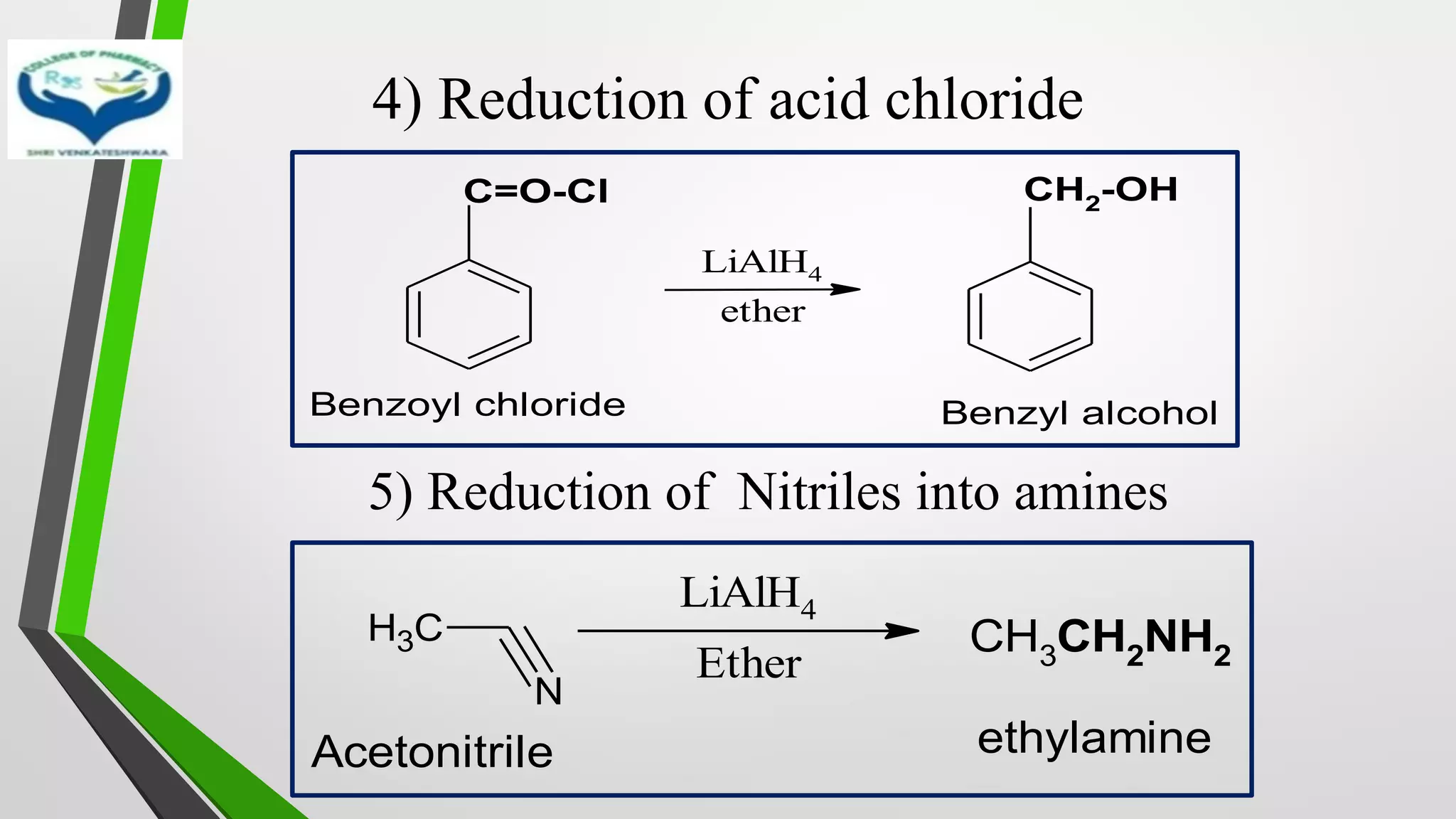
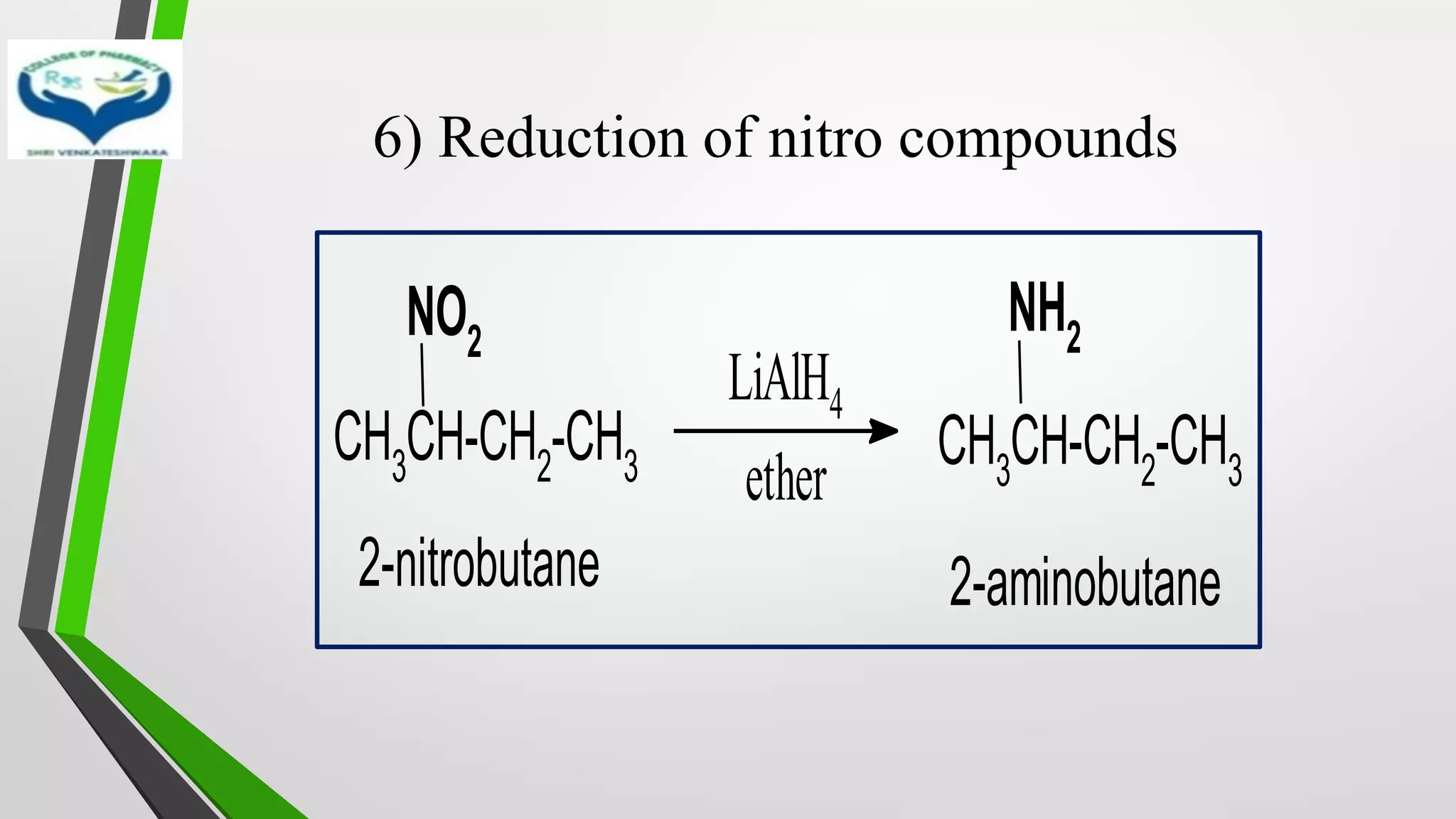
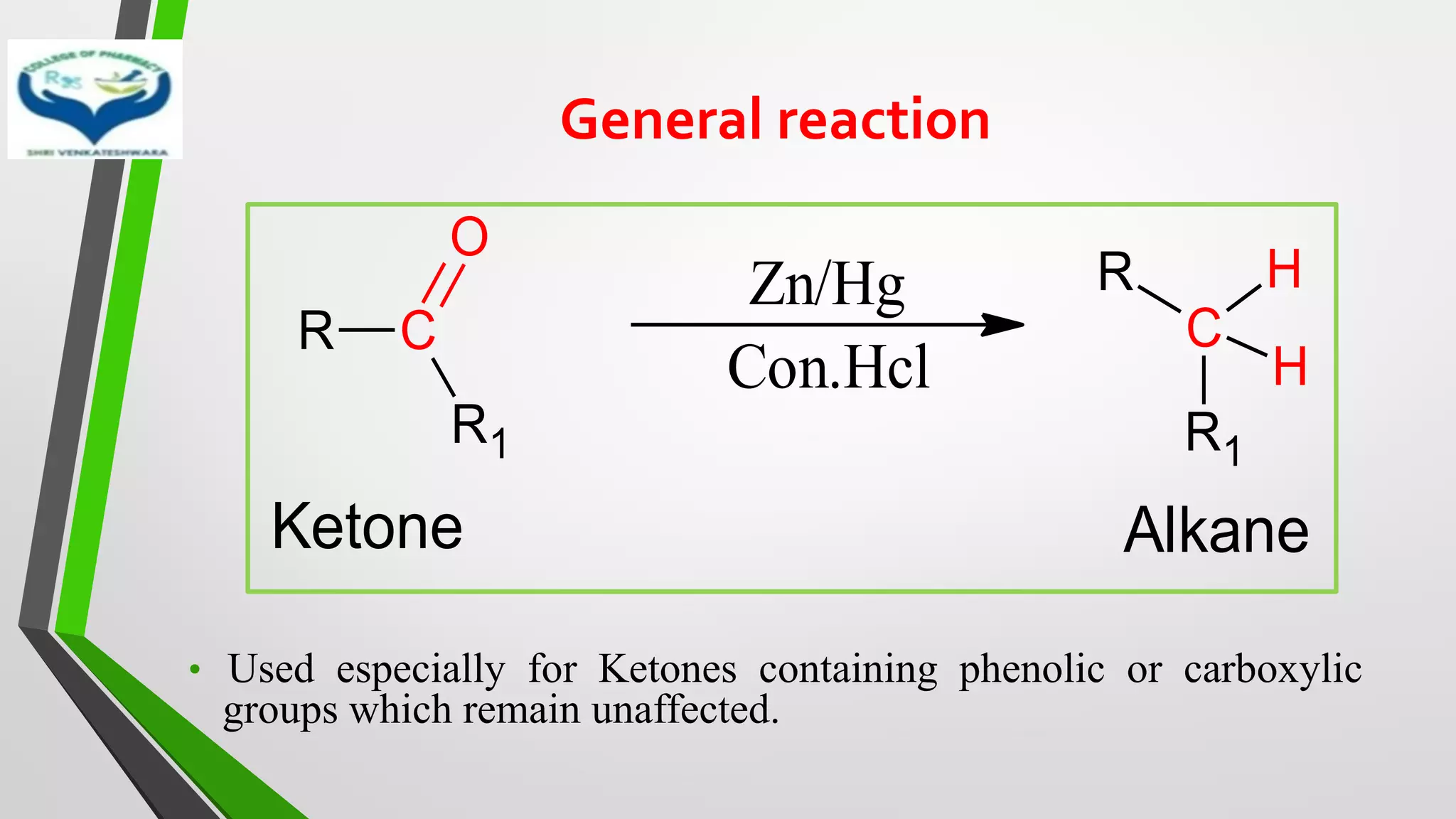
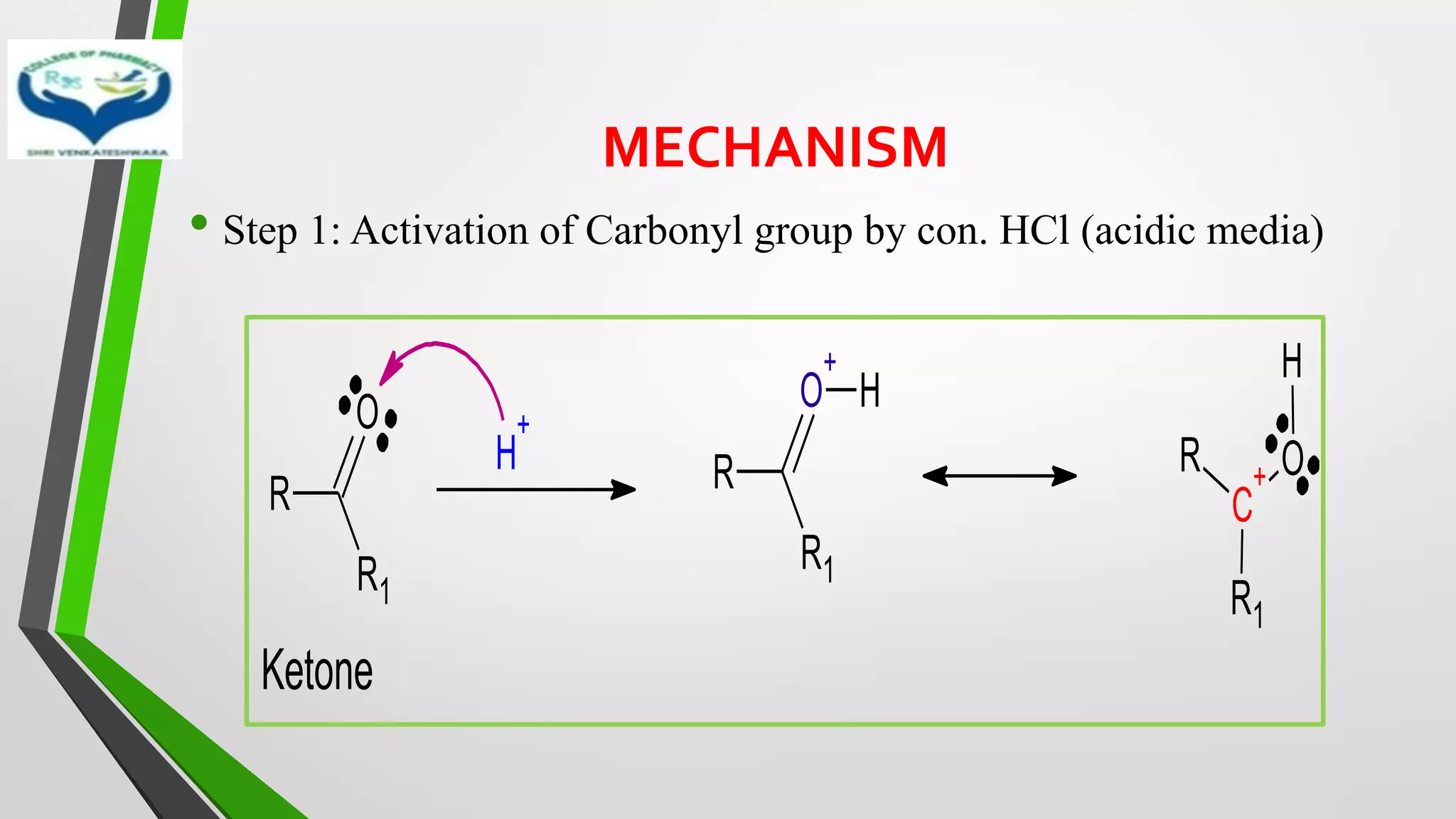
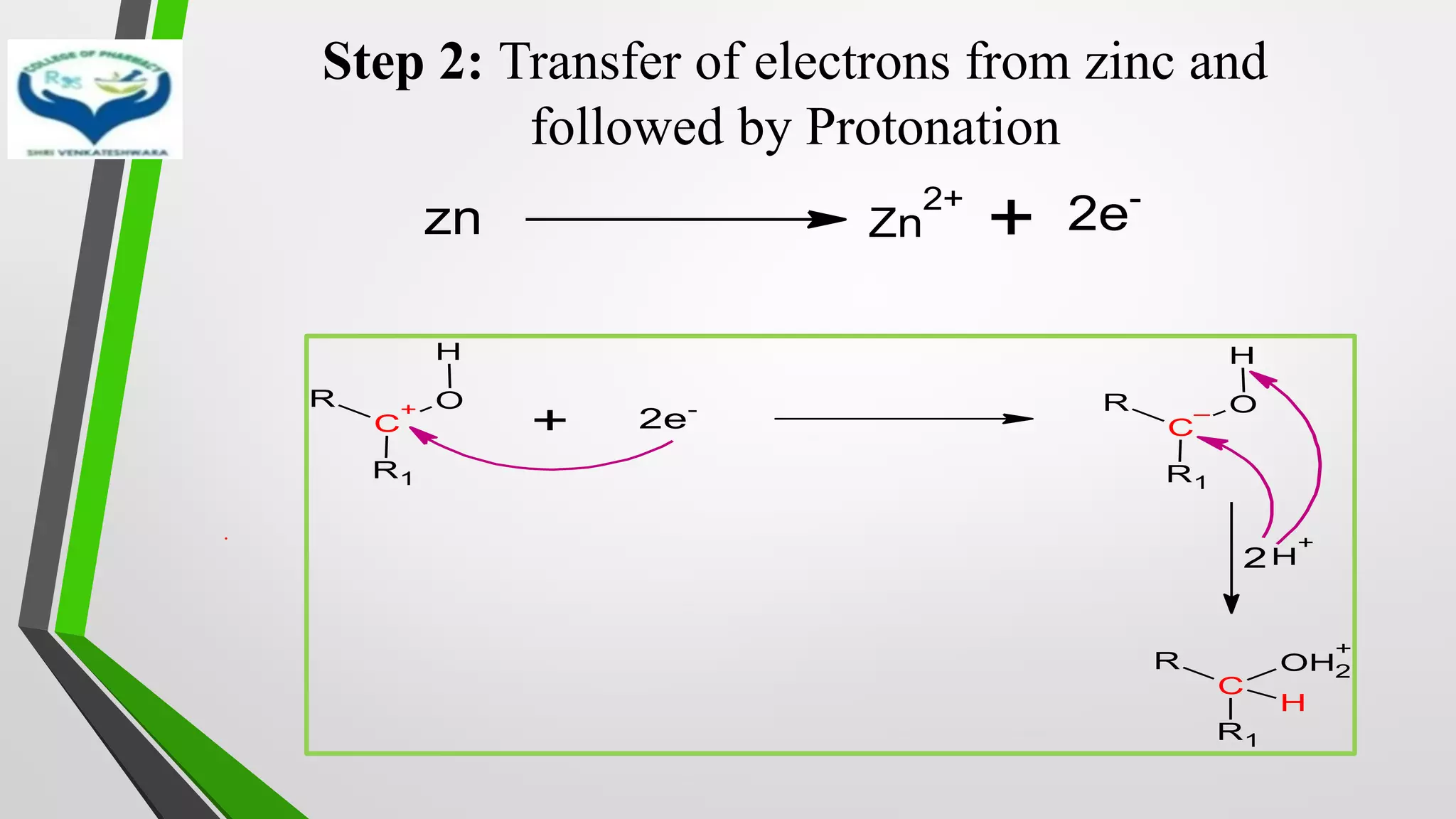
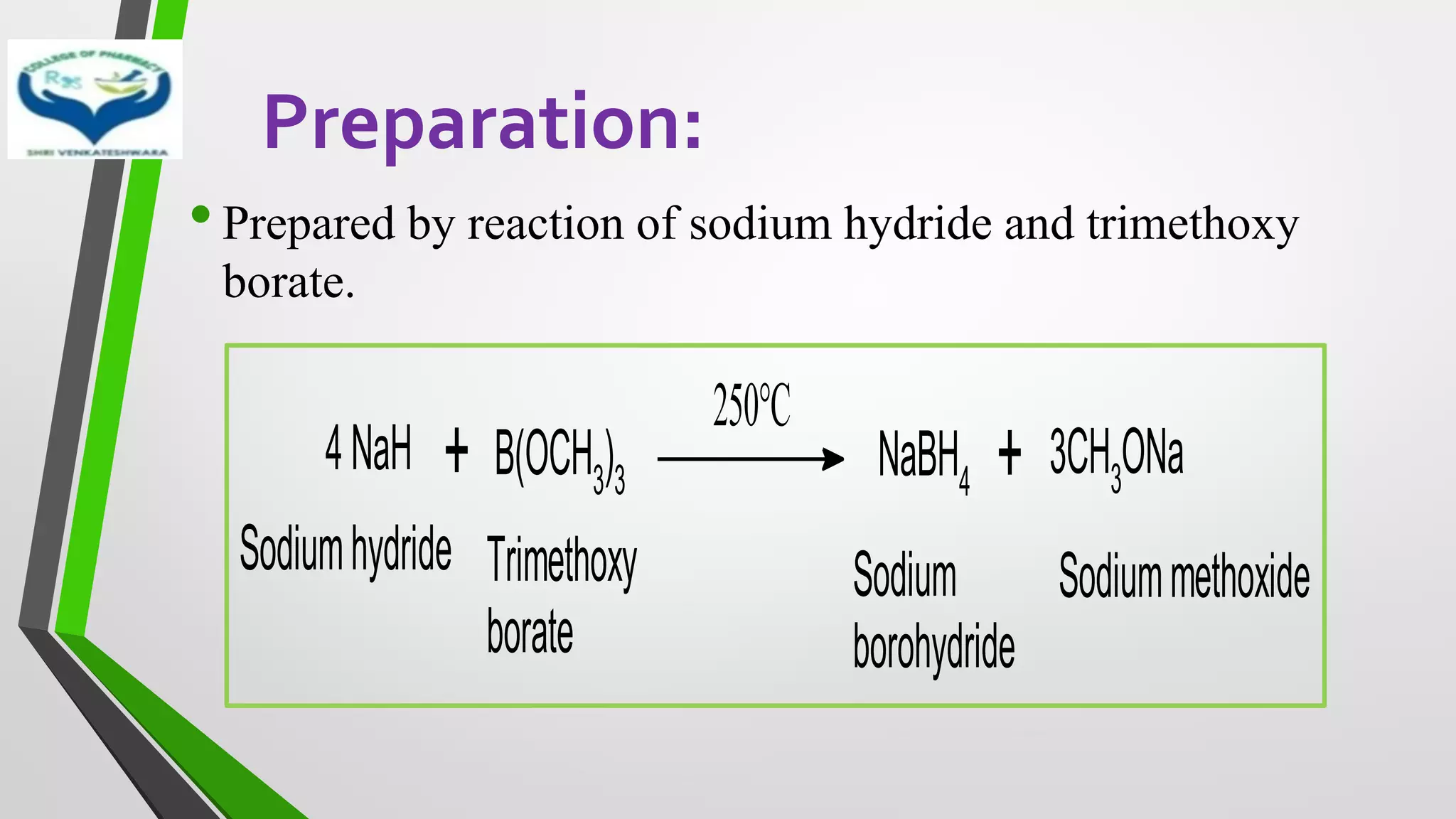
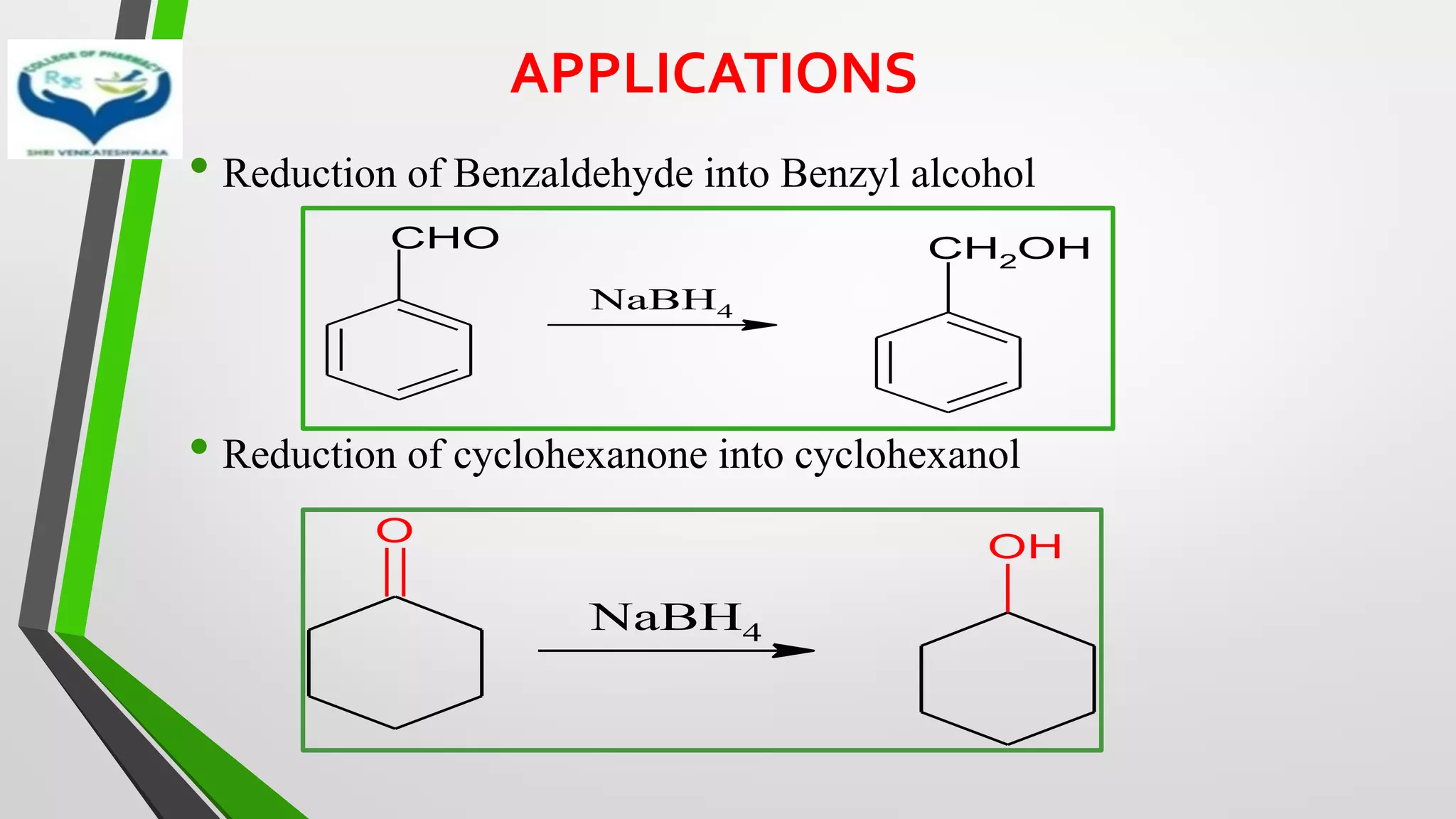
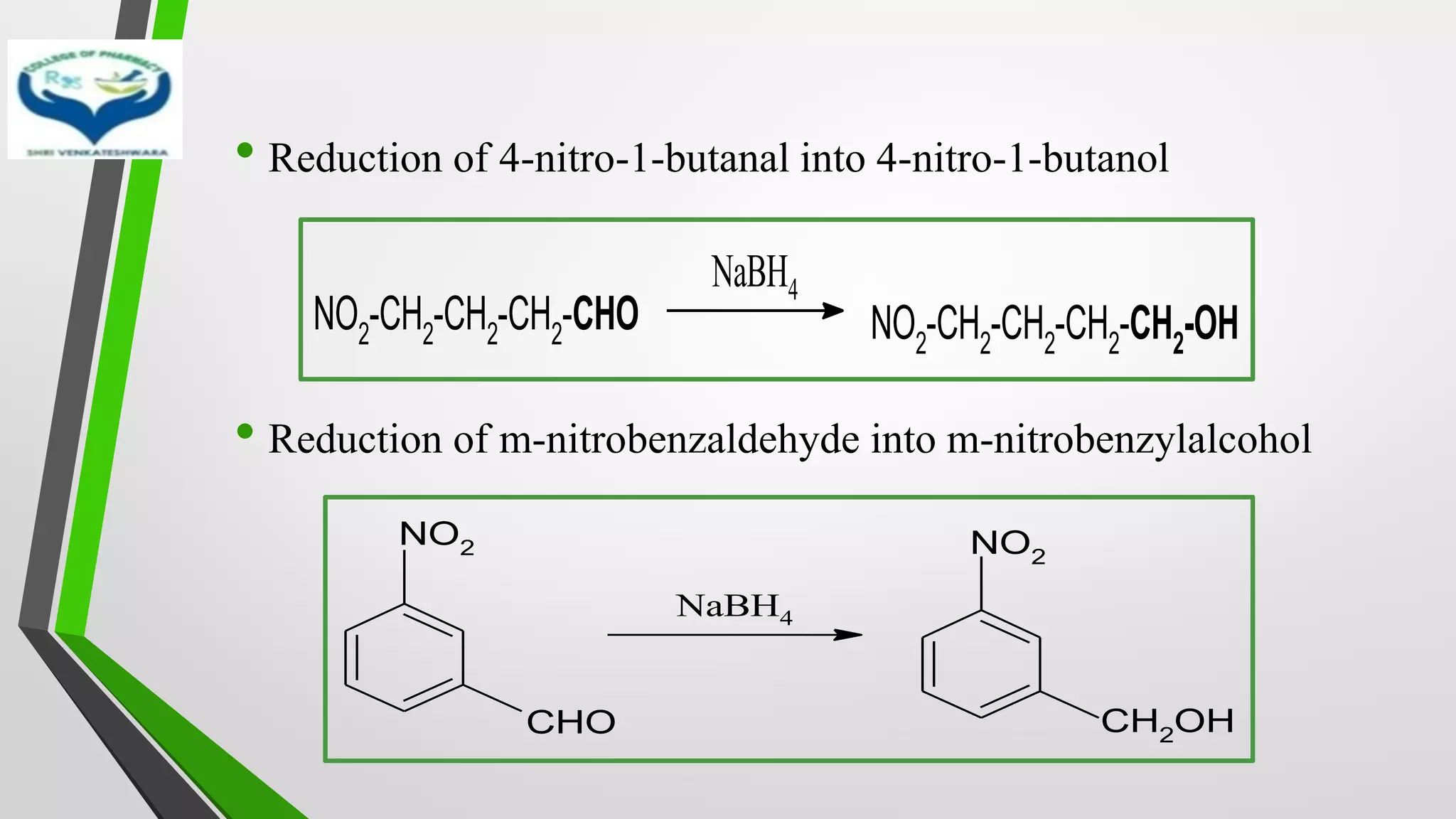
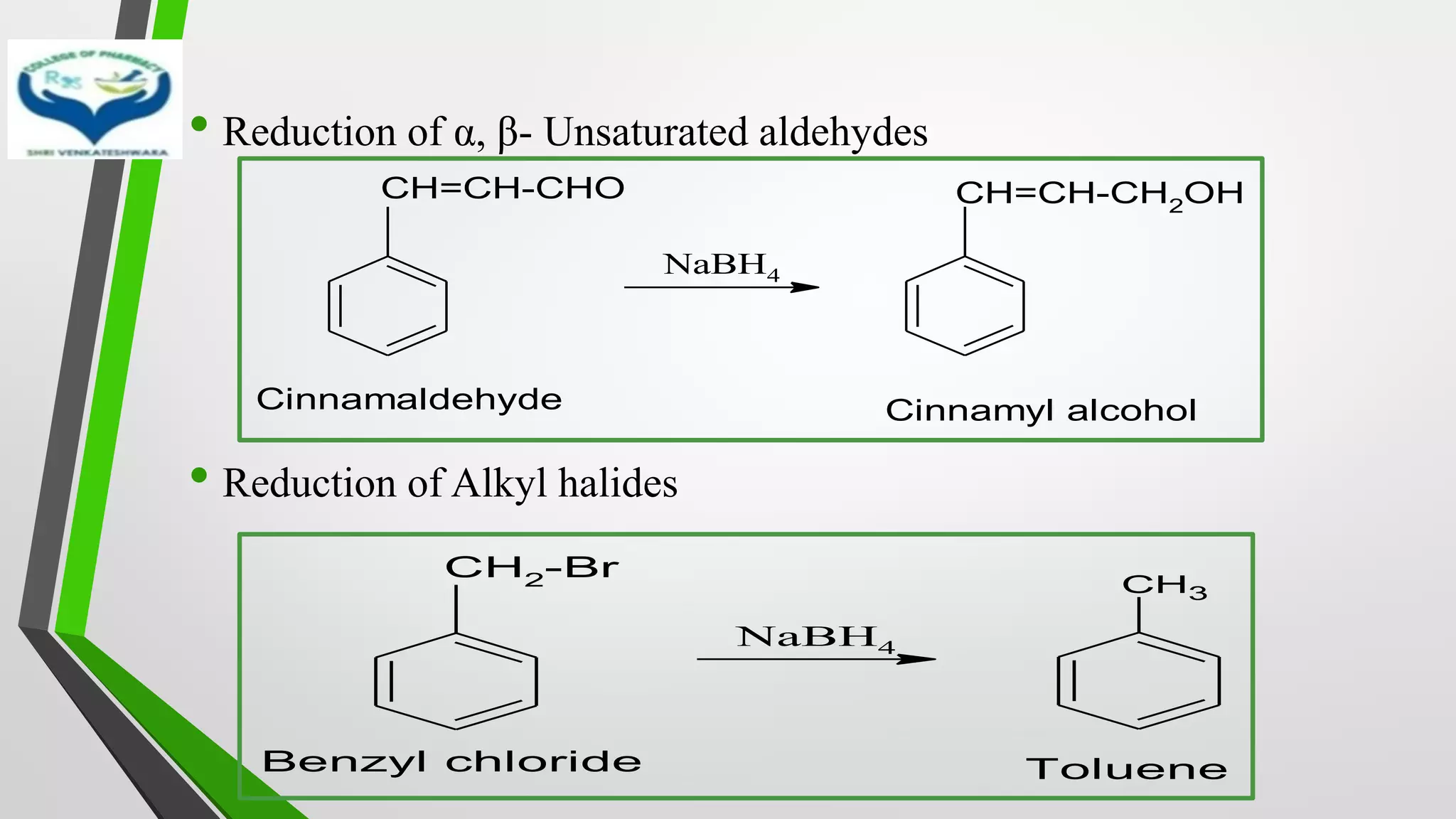
The document discusses two important metal hydride reductions - Clemmensen reduction and metal hydride reductions using sodium borohydride and lithium aluminium hydride. Clemmensen reduction involves the reduction of carbonyl groups to hydrocarbons using zinc amalgam and hydrochloric acid. Sodium borohydride is a mild reducing agent that reduces carbonyl groups to secondary alcohols. Lithium aluminium hydride is a strong reducing agent that can reduce a wide range of functional groups such as carbonyls, carboxylic acids, nitriles, and nitro groups to the corresponding alcohols or amines. Both sodium borohydride and lithium aluminium hydride reactions proceed by


![Definition
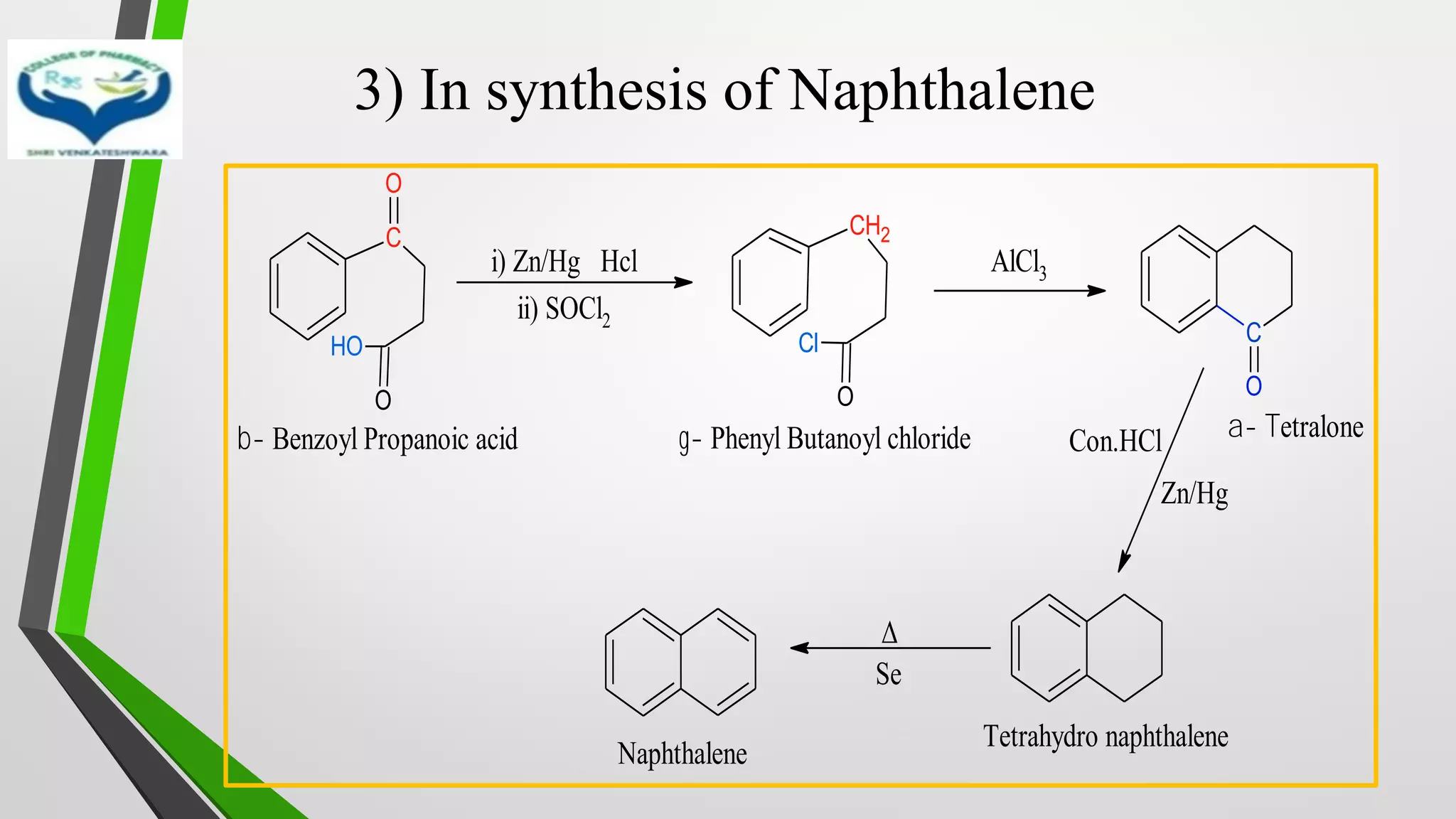
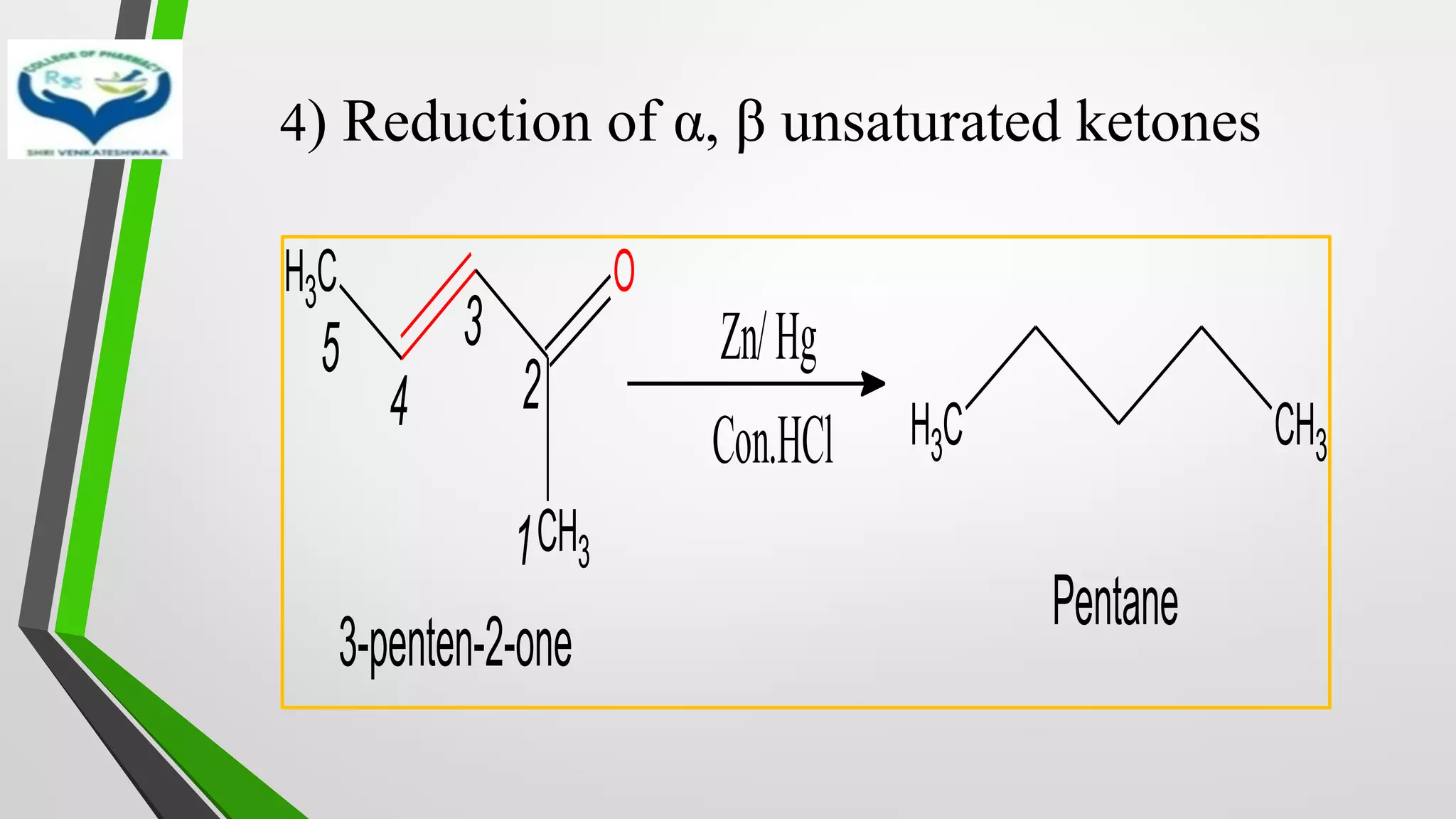
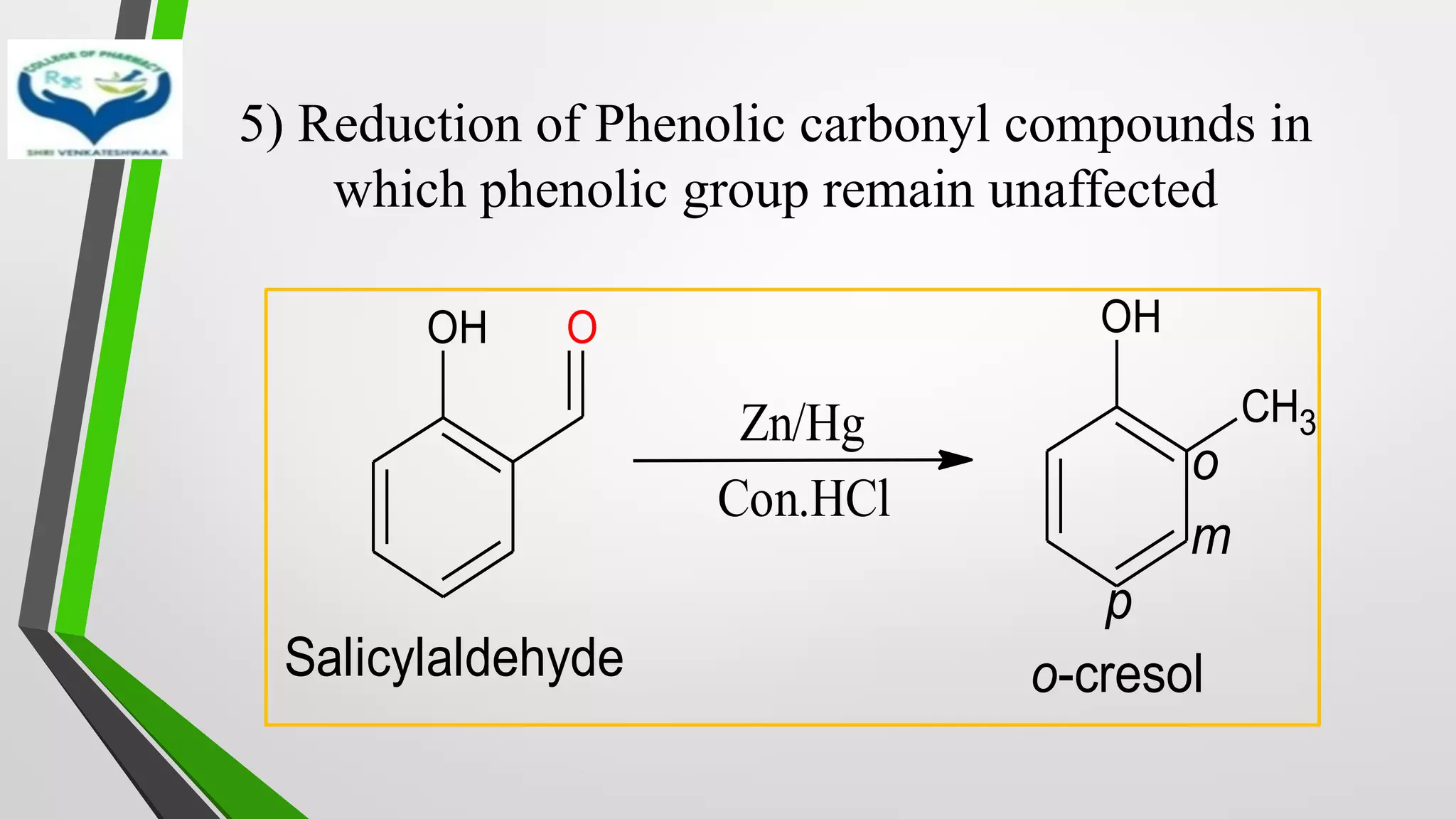
❖ Clemmensen Reduction was first reported by Clemmensen Park Davis
in 1913.
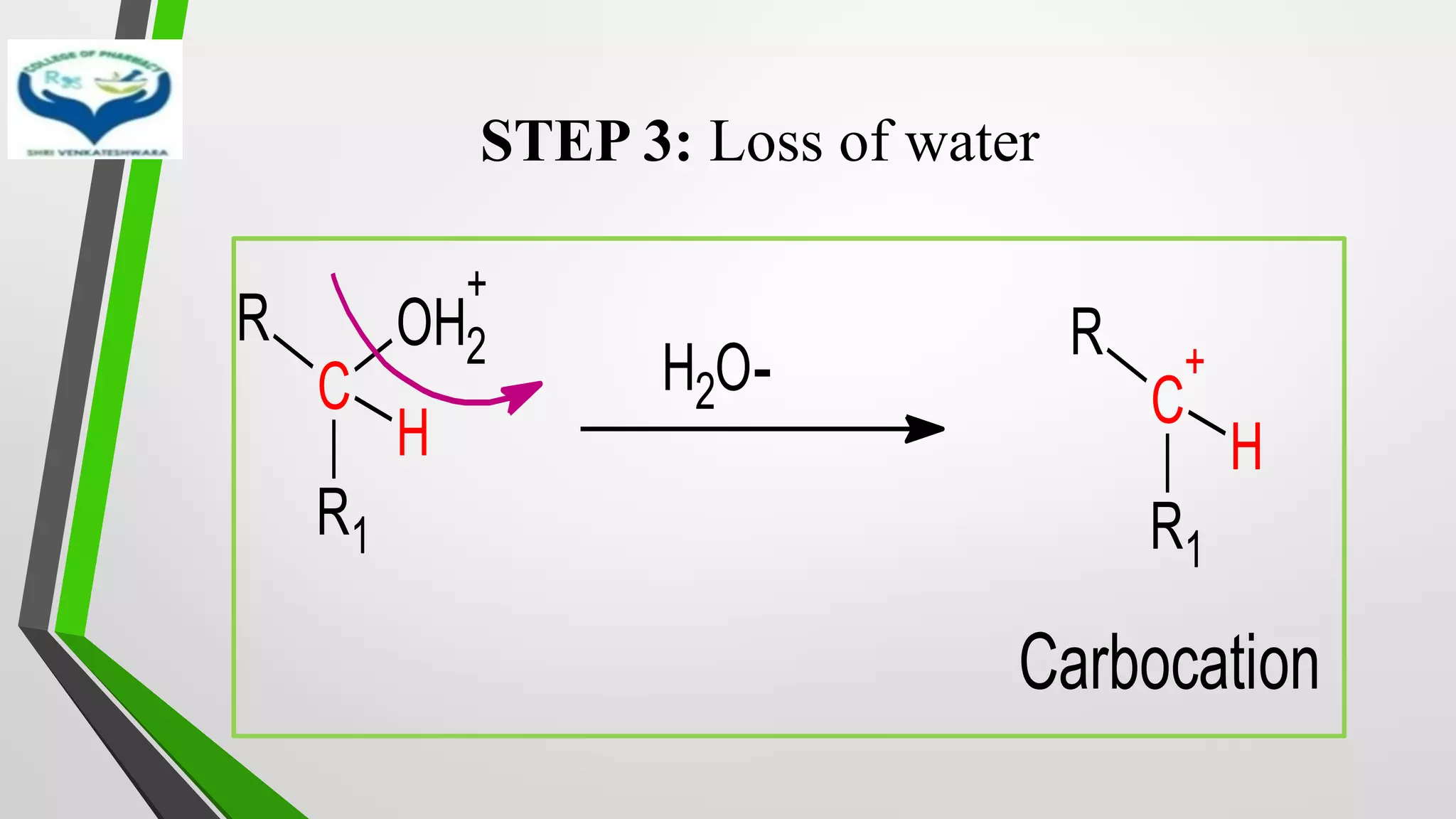
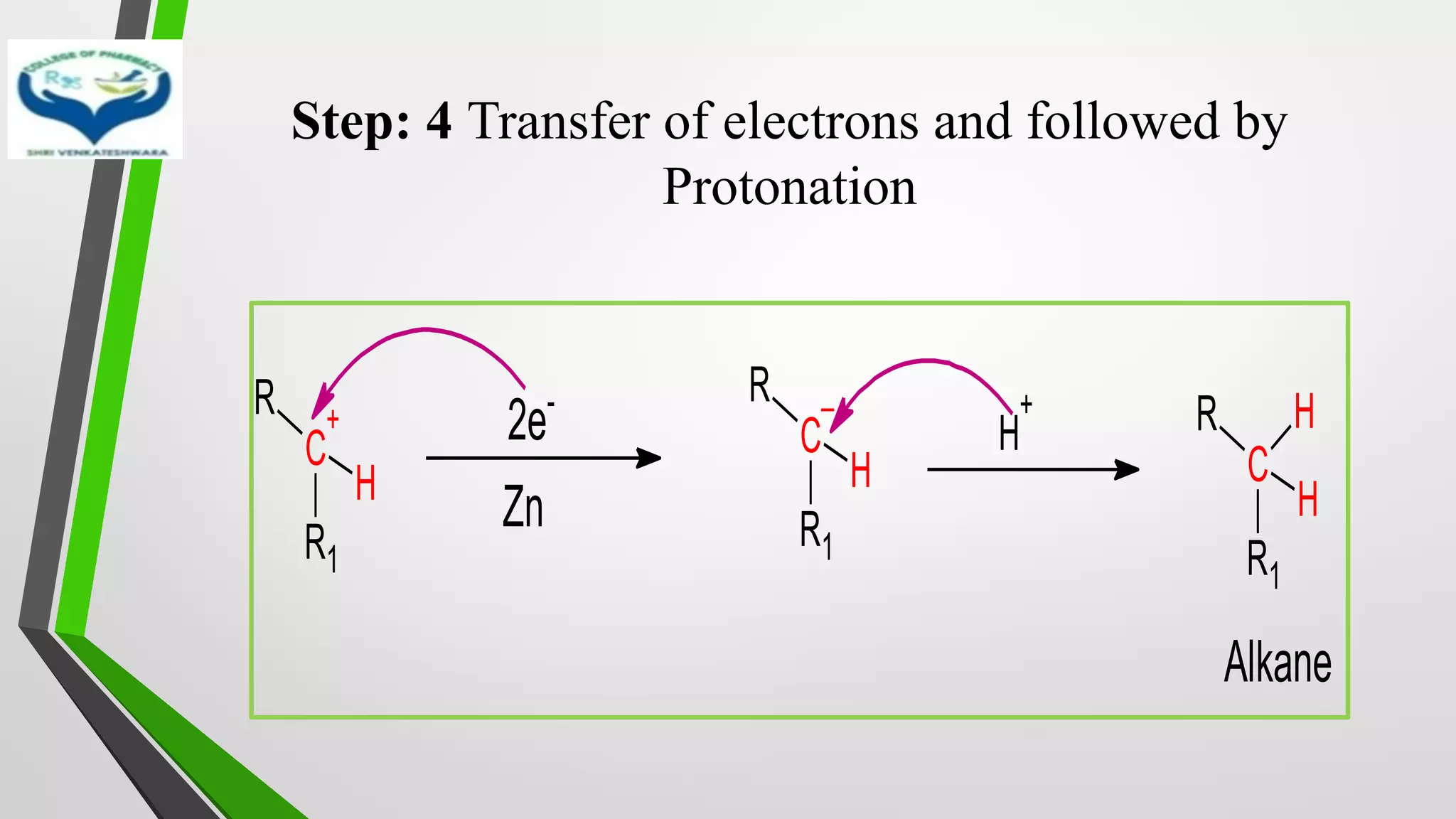
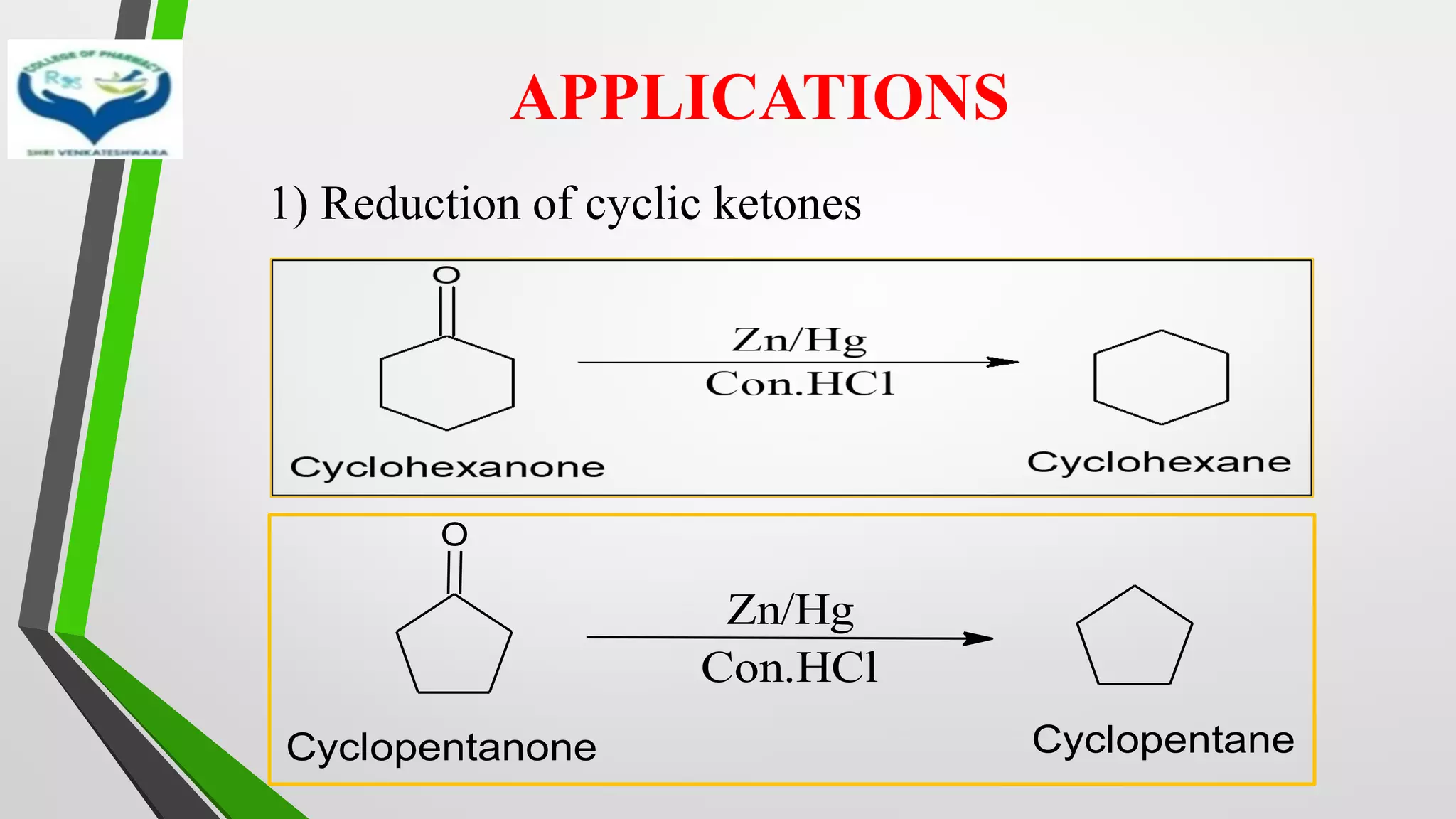
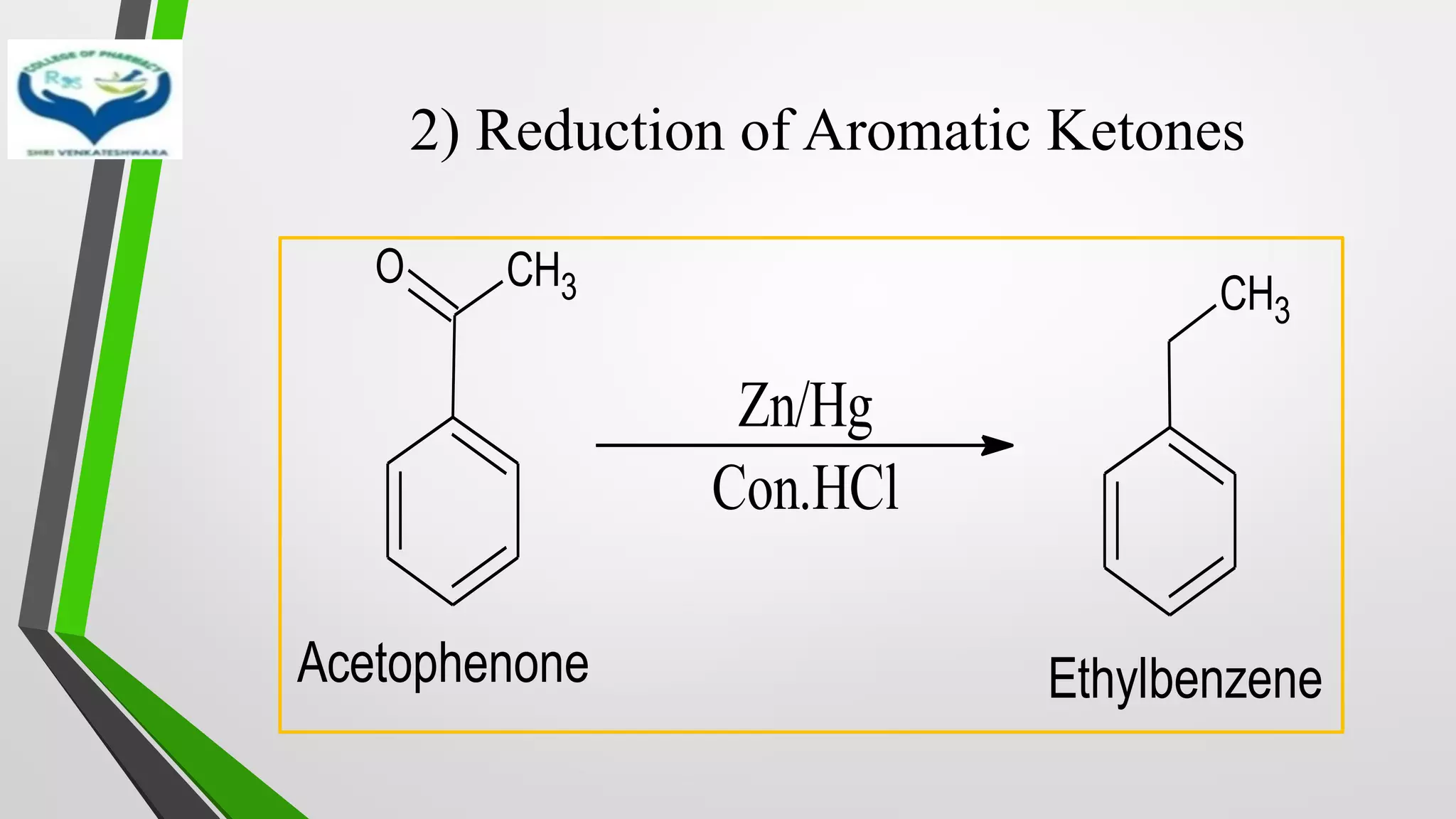
❖ The reduction of Carbonyl groups of aldehydes or ketones into
corresponding hydrocarbon or methylene group in presence of zinc
amalgam and excess con. HCl is known as Clemmensen reduction.
❖ALDEHYDES (-CHO)/ KETONES (-C=O)
[H]
❖ HYDROCARBONS (-CH2 )](https://image.slidesharecdn.com/reactionsofsyntheticimportance-210422060554/75/Reactions-of-synthetic-importance-3-2048.jpg)

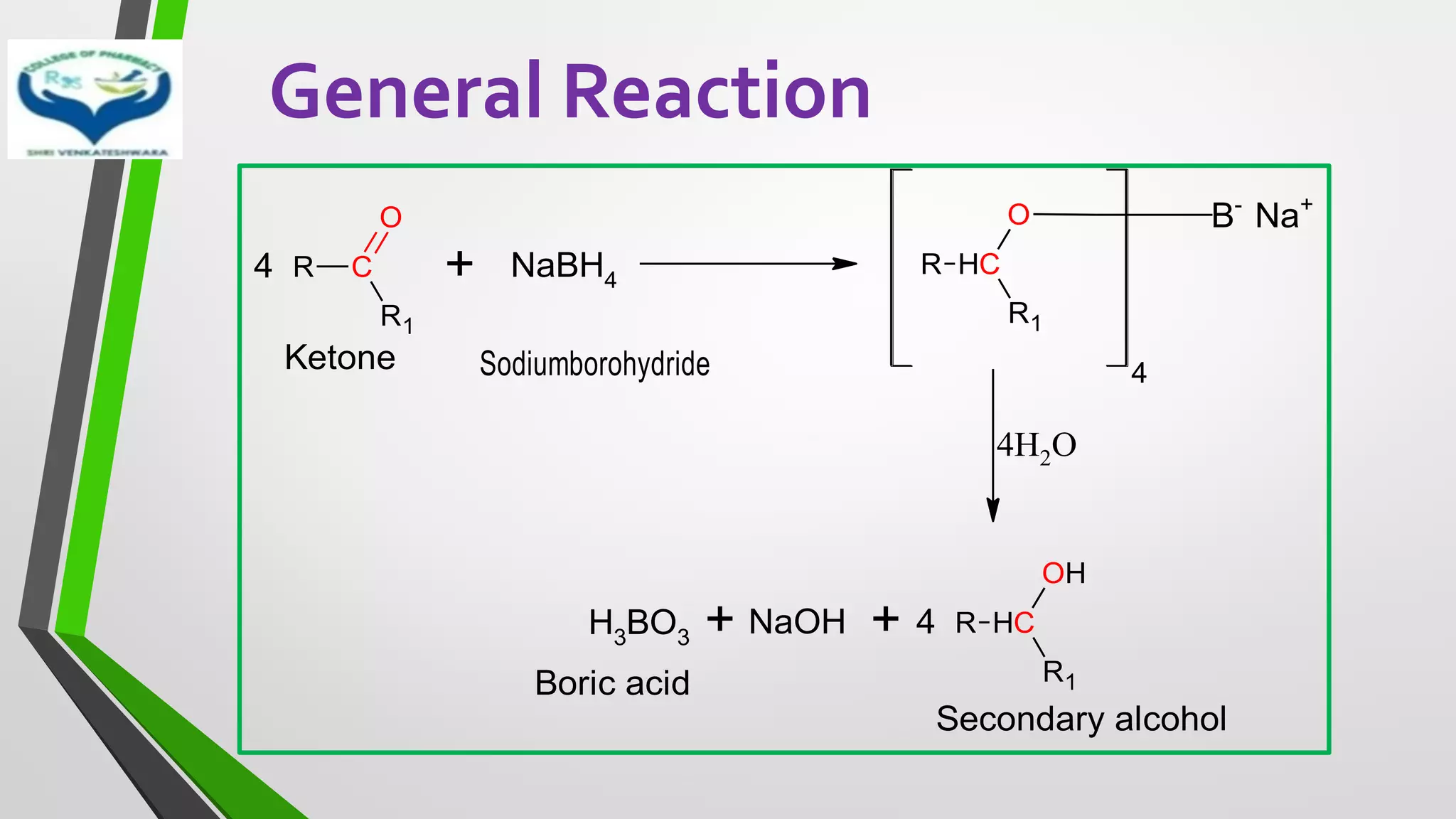
![INTRODUCTION
• Reduction of metal hydrides proceeds by transfer of hydride ion[H-] to the
substrate.
• Selectively reduce a number of functional groups such as
✓Carbonyl
✓Carboxylic acid
✓Nitro
✓Ester in presence C=C double bounds
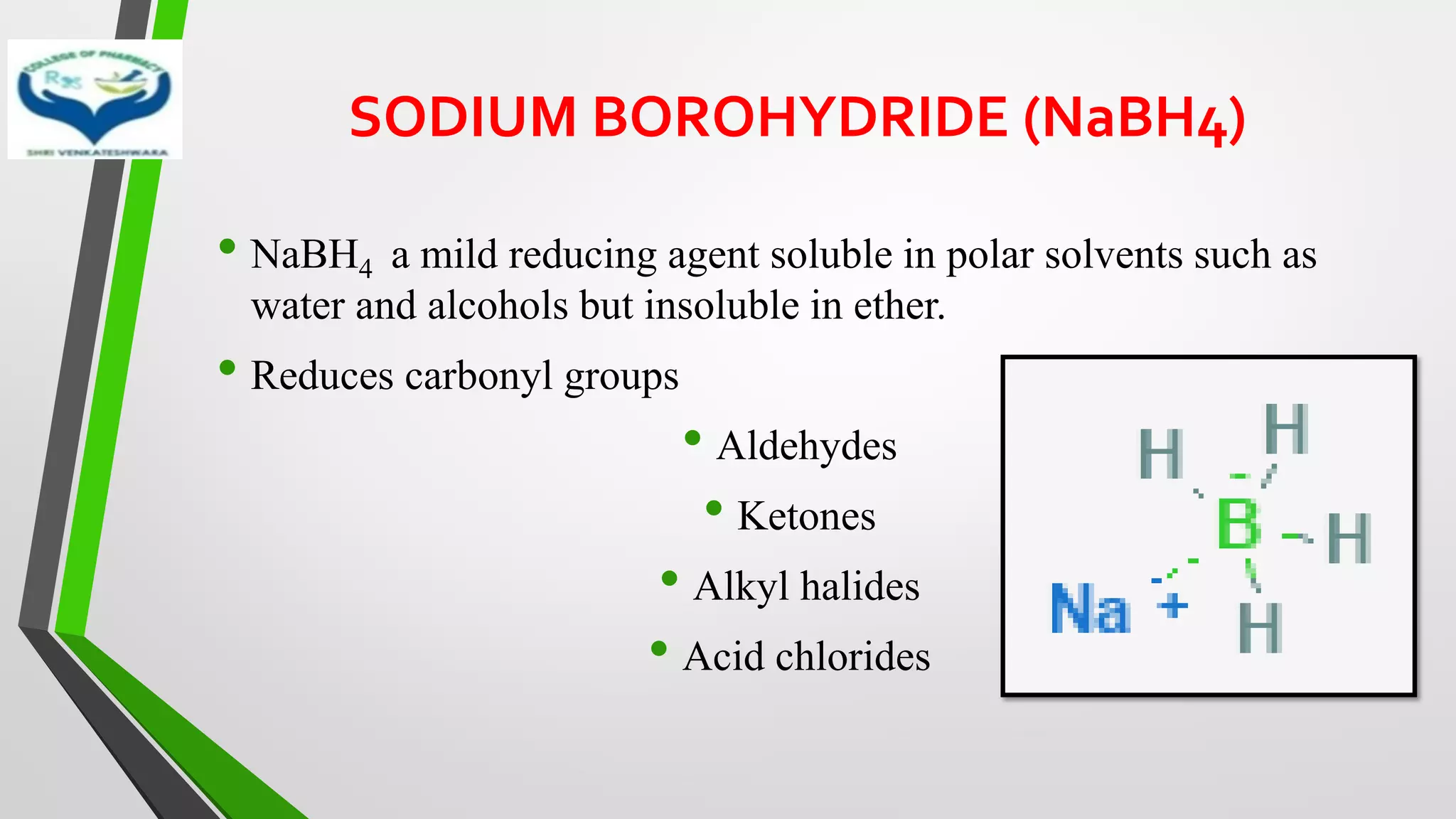
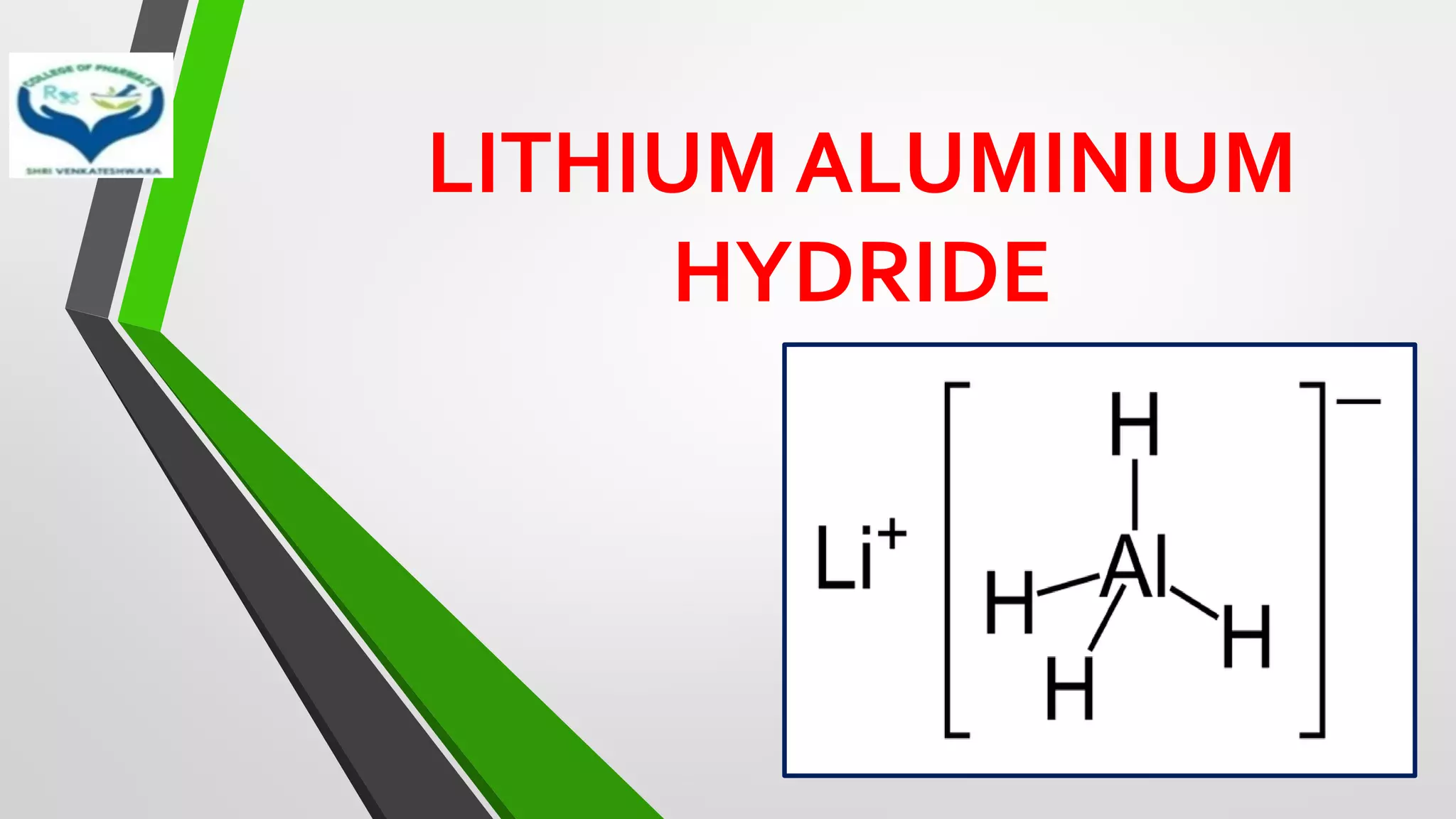
• Mostly used metal hydride reducing agents are sodium borohydride
(NaBH4) and lithium aluminium hydride (LiAlH4)
• LiAlH4 – Stong reducing agent
• NaBH4 – Mild reducing agent](https://image.slidesharecdn.com/reactionsofsyntheticimportance-210422060554/75/Reactions-of-synthetic-importance-15-2048.jpg)
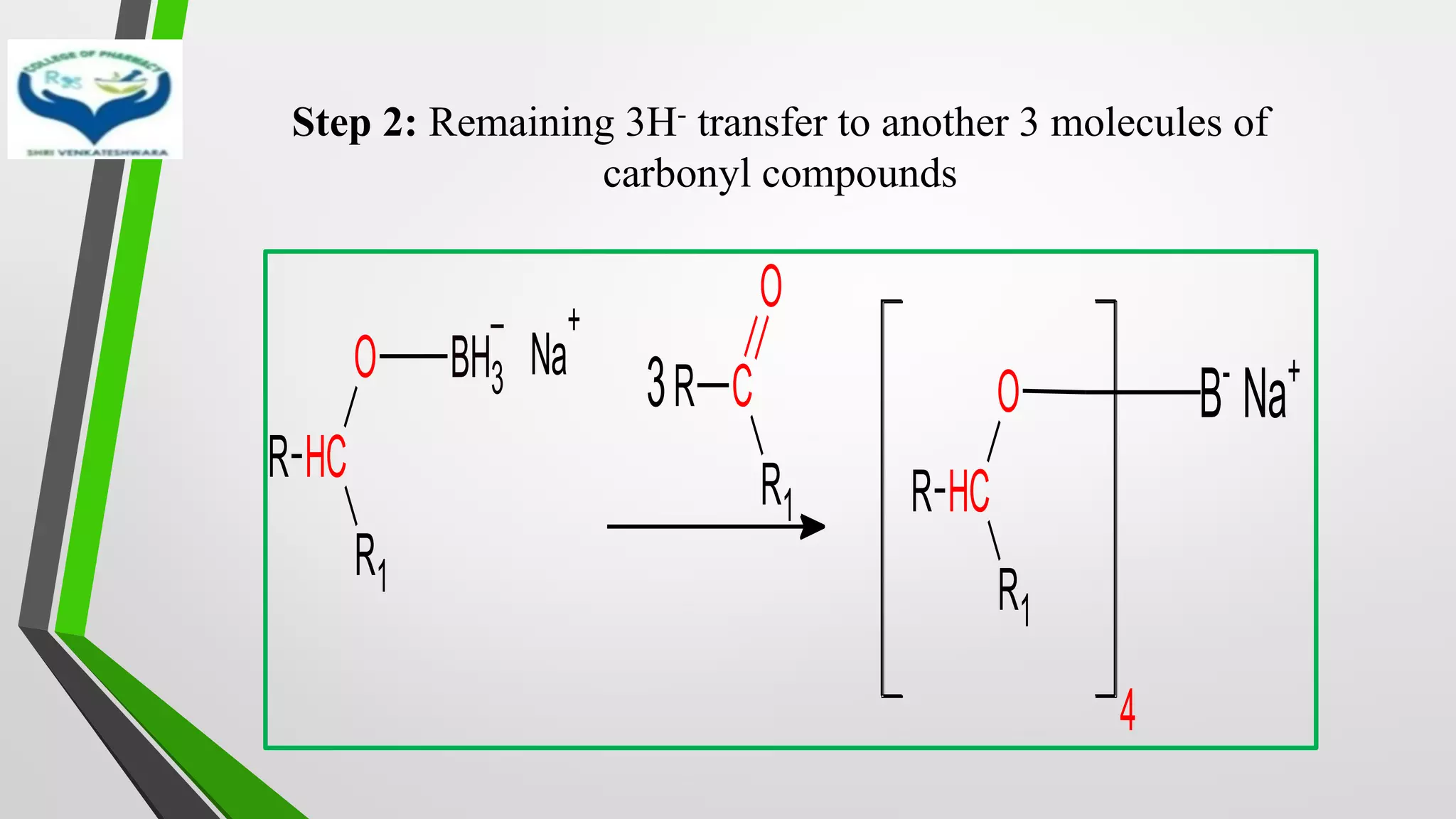
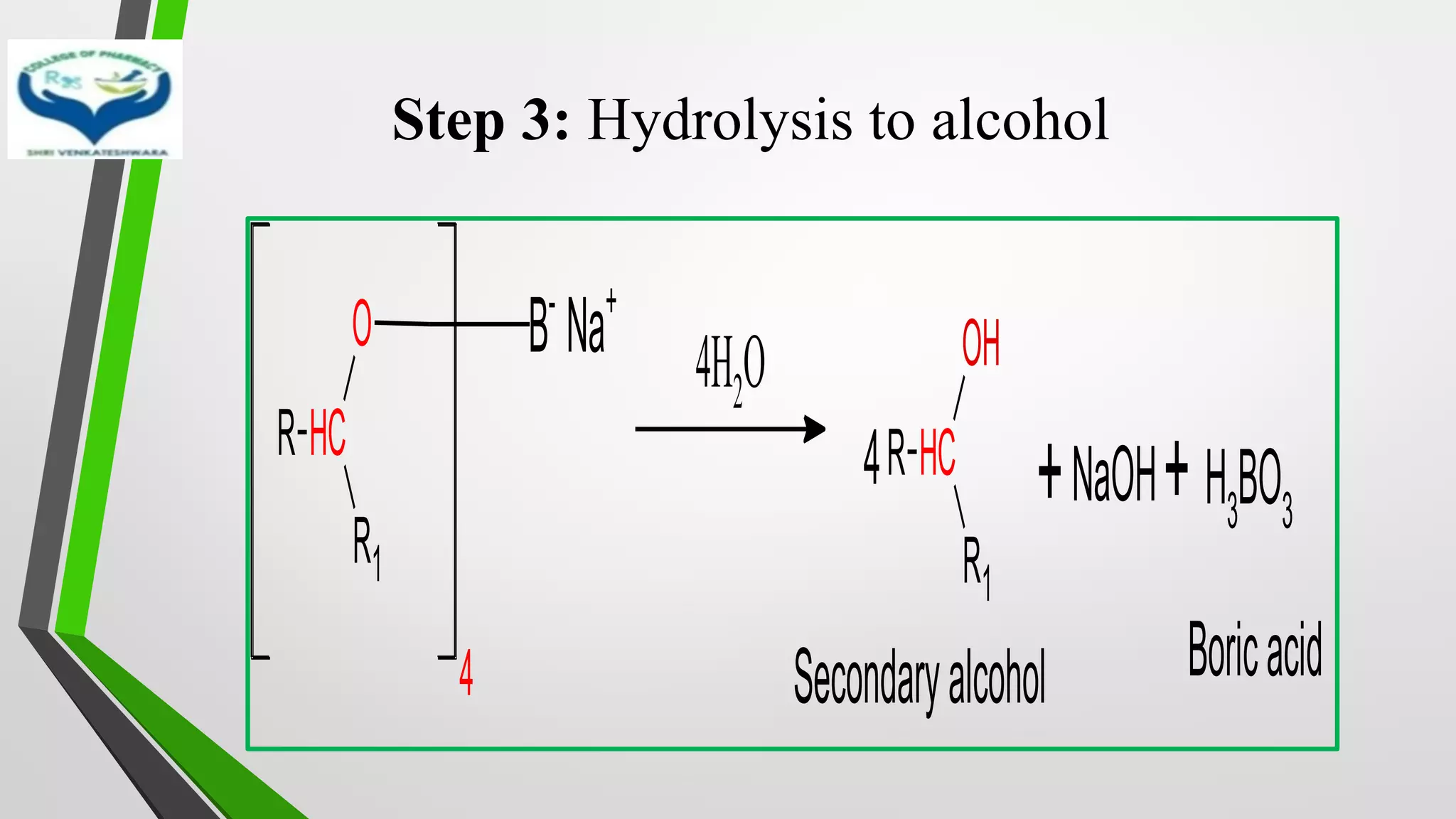
![Mechanism
• Step 1: Transfer of hydride ion [H-], powerful nucleophile from
NaBH4 to the carbonyl group with simultaneous binding of
carbonyl oxygen with boron
R C
R1
O
+ NaBH4
R C
R1
O
Na+
BH4
-
H-
R C
H
R1
O BH3
–
Na
+
Complex](https://image.slidesharecdn.com/reactionsofsyntheticimportance-210422060554/75/Reactions-of-synthetic-importance-19-2048.jpg)




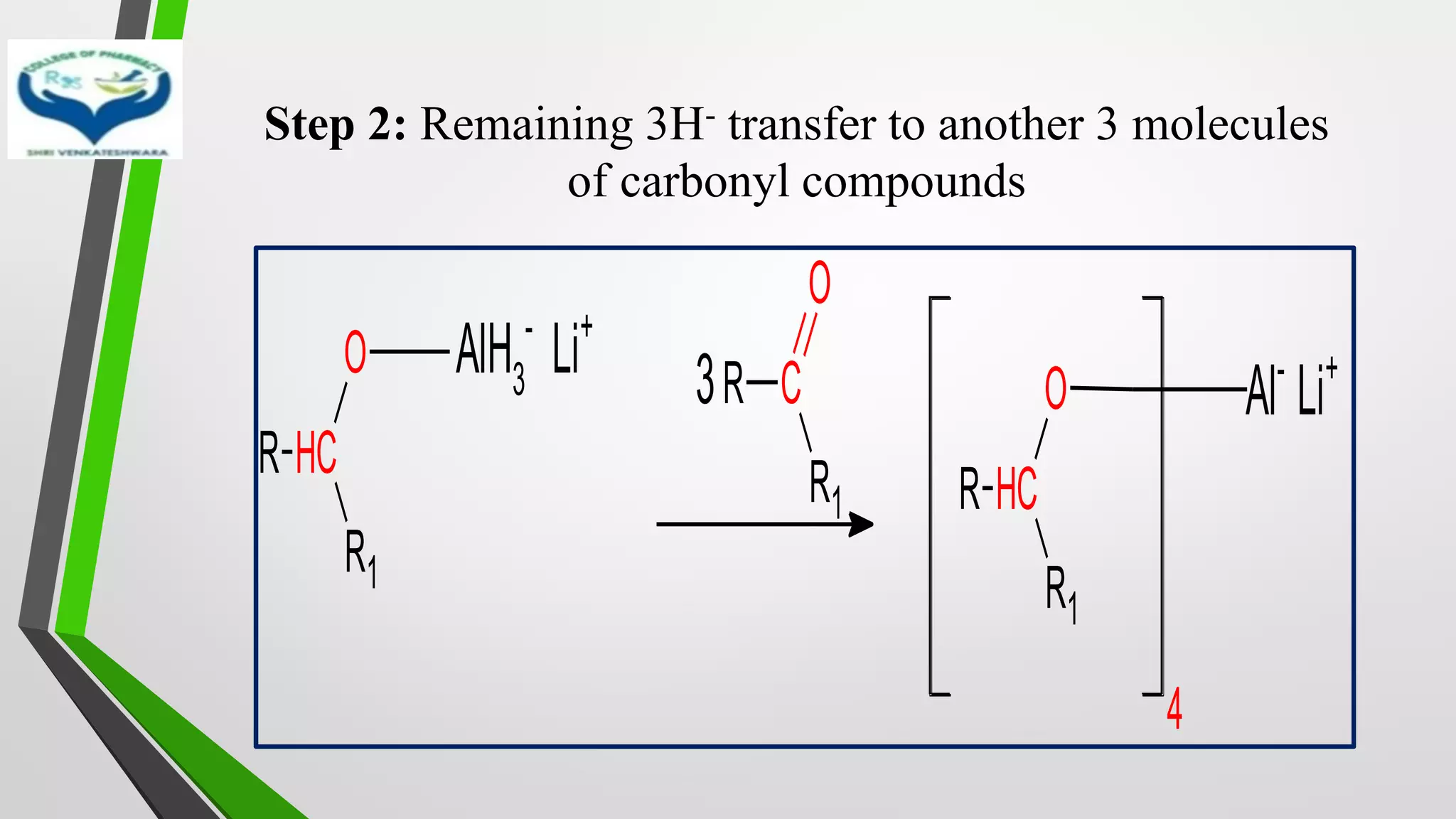
![MECHANISM
• Step 1: Transfer of hydride ion [H-], powerful nucleophile from
LiAlH4 to the carbonyl group with simultaneous binding of
carbonyl oxygen with boron
R C
R1
O
+ LiAlH4
R C
R1
O
Li+
AlH4
-
H-
R C
H
R1
O
Complex
AlH3
-
Li+](https://image.slidesharecdn.com/reactionsofsyntheticimportance-210422060554/75/Reactions-of-synthetic-importance-30-2048.jpg)