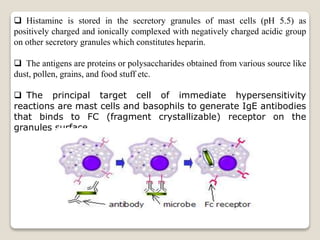
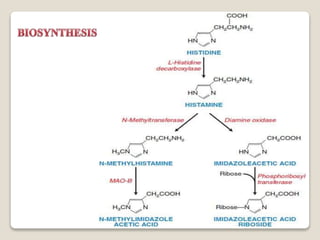
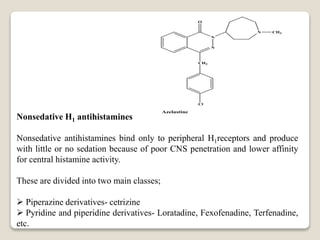
- Histamine was first identified in 1911 and is found throughout the human body. It is synthesized from the amino acid histidine and stored in mast cell granules.
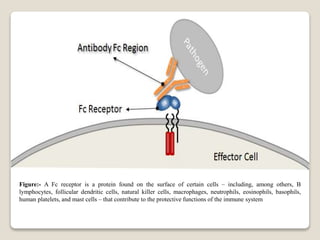
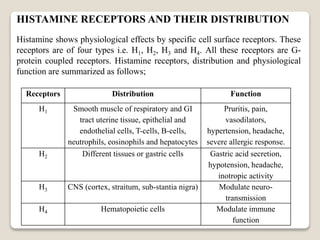
- Histamine binds to four receptor types (H1, H2, H3, H4) and is involved in various physiological processes like smooth muscle contraction and vasodilation. It causes allergic symptoms.
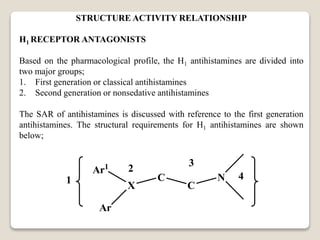
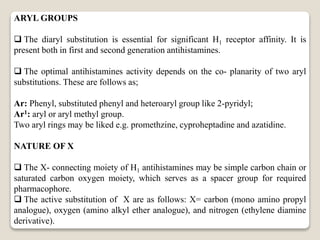
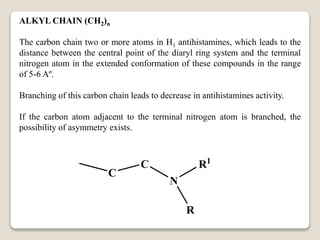
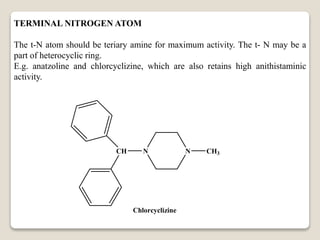
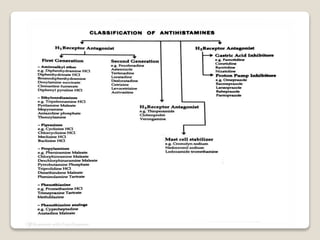
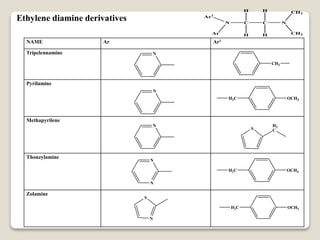
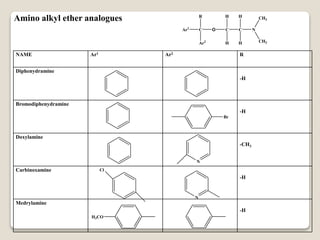
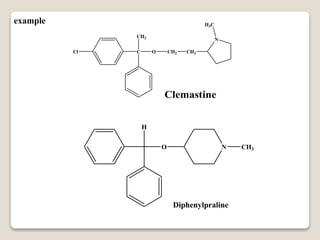
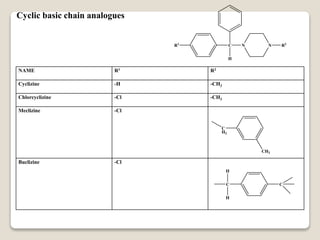
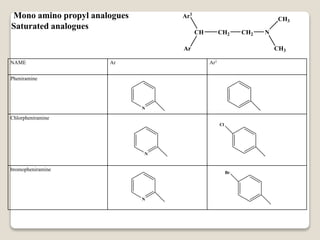
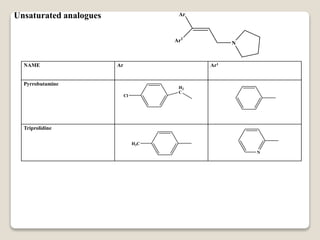
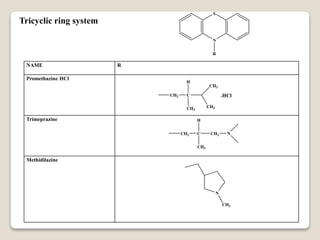
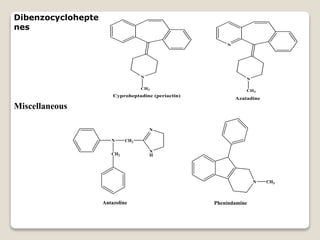
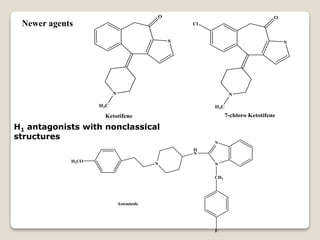
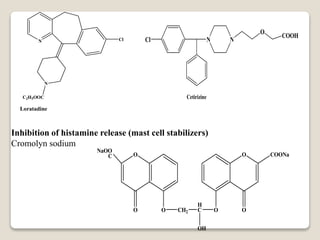
- Antihistamines work by blocking H1 and/or H2 receptors. First generation antihistamines are sedating while second generation ones are non-sedating. Structural requirements for antihistamine activity include diaryl substitutions, an optimal distance between rings, and a tertiary amine group.
![ Histamine was first identified in 1911 by Barger and Dale. Anti-histamines are
the drugs which counteract the actions of histamine in the body.
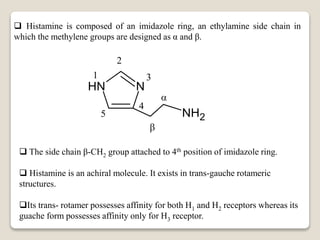
Histamine [2-(imidazol-4-yl) ethylamine], which is biosynthesized by
decarboxylation of the basic amino acid histidine, is found in all organs and
tissues of the human body.
NH
N
H
H H
NH2
O
OH
2-amino-3-(1H-imidazol-4-yl)propanoic
acid
Histidine
Histamine
Histidine decarboxylation
-CO2
NH
N
H
H H
H
NH2
2-(1H-imidazol-4-yl)ethan-1-amine](https://image.slidesharecdn.com/antihisminicagents-200825093843/85/Antihisminic-agents-2-320.jpg)