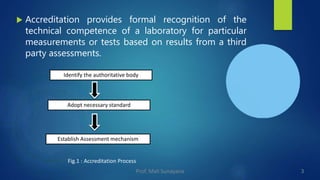
Accreditation of laboratories is a process through which an authorized body examines and certifies the competence and quality systems of a laboratory based on predefined standards. NABL is India's national accreditation body that specifies the requirements for testing and calibration laboratories to be considered technically competent. The accreditation process involves identifying standards, assessment procedures, training, documentation, internal audits, management reviews, and a final certification audit. Laboratories must prepare for accreditation by training personnel, documenting procedures, implementing quality control, participating in proficiency testing, and addressing any gaps before applying to NABL for assessment.