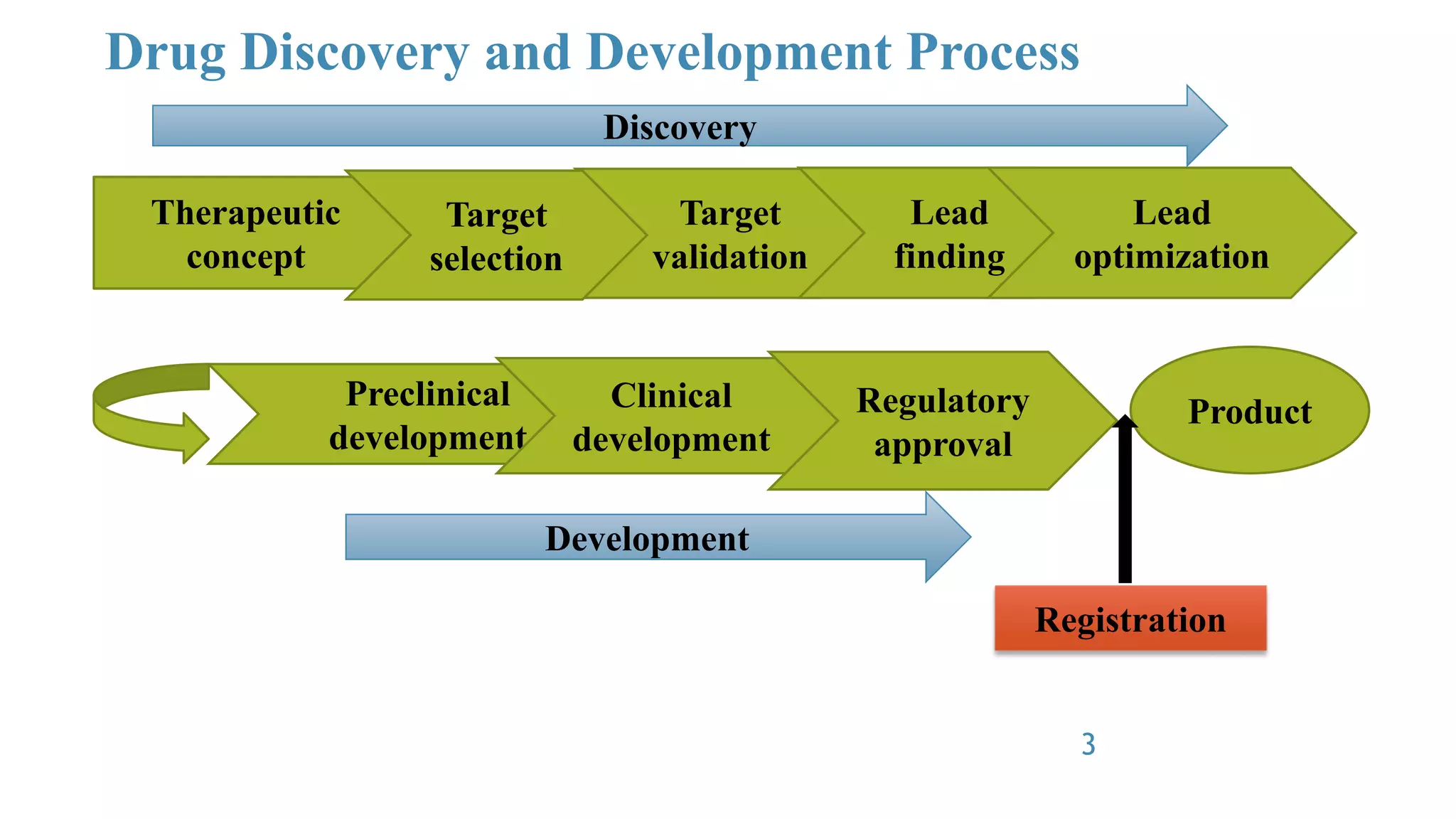
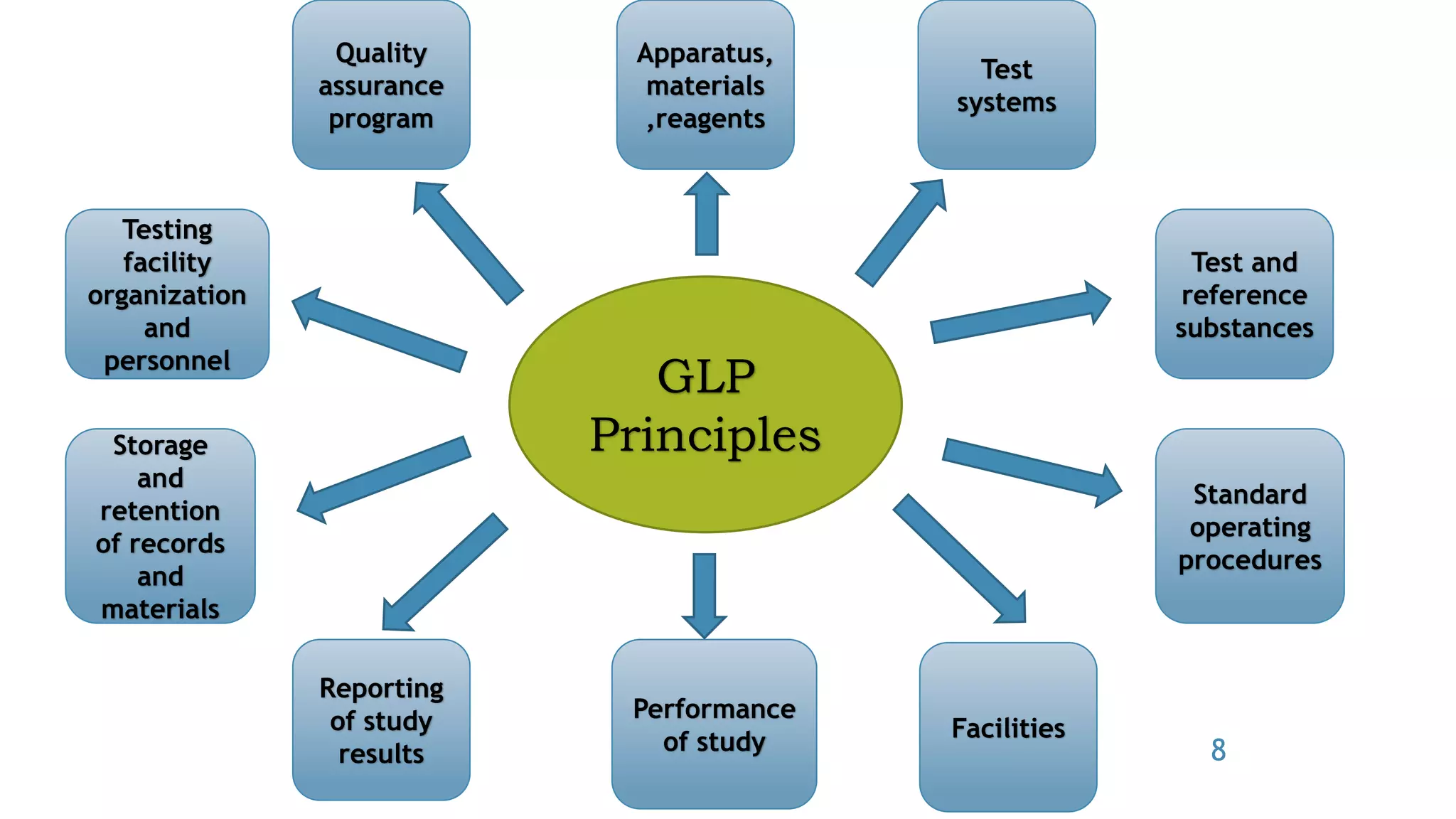
The document discusses Good Laboratory Practice (GLP) guidelines. It notes that in the 1970s, the FDA discovered many cases of poor laboratory practices and fraudulent activities in the US. This led to the establishment of GLP principles to ensure reliable and high-quality non-clinical safety studies. GLP guidelines cover facilities, equipment, standard operating procedures, personnel training, record-keeping, and quality assurance programs. The goal of GLP is to ensure that experimental data accurately reflect the results and that studies are conducted in accordance with the principles of good laboratory practice.