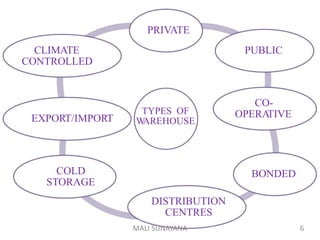
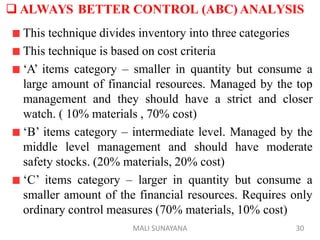
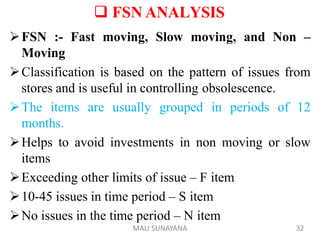
The document discusses good warehousing practices for pharmaceutical products. It outlines the WHO guidelines which include 6 key areas: 1) personnel requirements to properly staff and train warehouse employees, 2) premises and facility standards regarding storage areas, conditions and security, 3) storage requirements such as documentation, labeling and container standards, 4) handling returned goods with established procedures, 5) dispatch and transportation standards to maintain product quality during delivery, and 6) product recall protocols for defective goods. Following good warehousing practices helps ensure the quality of pharmaceutical products is maintained throughout the storage and distribution process.