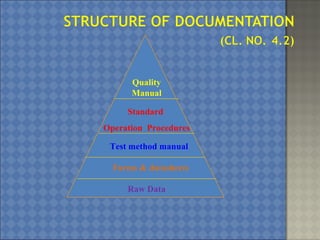
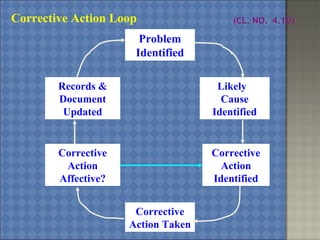
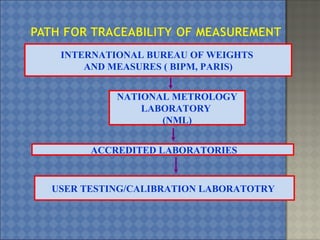
This document discusses the key requirements of ISO/IEC 17025 for testing and calibration laboratories. It covers management requirements, technical requirements, quality management systems, personnel requirements, accommodation and environmental conditions, test and calibration methods, equipment, measurement traceability, sampling, handling of test and calibration items, quality control, reporting of results, subcontracting, and opinions and interpretations. The main points are that ISO/IEC 17025 establishes the requirements for laboratories to demonstrate they are technically competent and able to produce valid results, laboratories must implement a quality management system, and personnel performing testing and calibrations must be qualified.