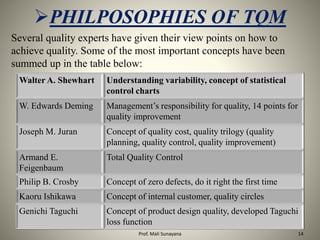
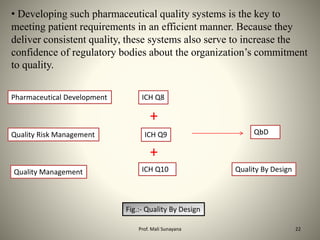
This document provides an overview of Total Quality Management (TQM) principles, detailing its history, key elements, and specific applications in the pharmaceutical industry. It explains the importance of TQM in enhancing product quality, customer satisfaction, and organizational efficiency, while also discussing the concept of Quality by Design (QbD) in drug development. Additionally, it lists influential figures in the quality movement and their contributions to contemporary quality practices.