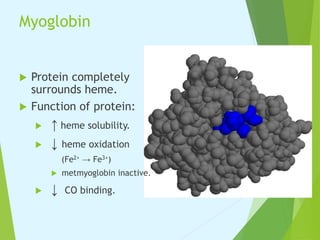
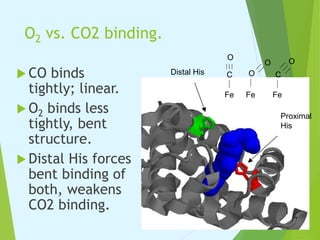
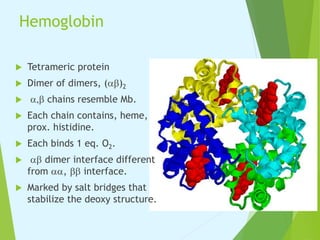
Myoglobin and hemoglobin are proteins that transport oxygen in the body. Myoglobin transports and stores oxygen in muscles. It has a globin protein that surrounds a heme group, protecting it and allowing oxygen binding. Hemoglobin transports oxygen in blood and differs from myoglobin in being a tetramer of four globin chains that bind oxygen cooperatively. The binding of oxygen to one chain increases the affinity of the other chains. This allows hemoglobin to efficiently load and release oxygen in the lungs and tissues respectively.






![Cooperative regulation
Hb oxygen binding:
Start binding with given affinity in deoxy state, subsequent binding
enhances affinity.
Defines positive cooperative regulation.
Only seen in multi-domain proteins.
Hill coefficient ~ number of interacting subunits. Advantage: binding
is more sensitive to small changes in [ligand].](https://image.slidesharecdn.com/mbhb1-210222040707/85/Myoglobin-and-Hemoglobin-11-320.jpg)



![2,3-BPG
Side product of
glycolysis.
indicates active
respiration, need O2.
Binds cationic region in
T-form.
Favors deoxy, releases O2
to tissues.
[2,3-BPG] is high,
responsible for observed
pO2,50 of 27 mm Hg.
stripped Hb has pO2, 50 ~
8 mm Hg.](https://image.slidesharecdn.com/mbhb1-210222040707/85/Myoglobin-and-Hemoglobin-16-320.jpg)

