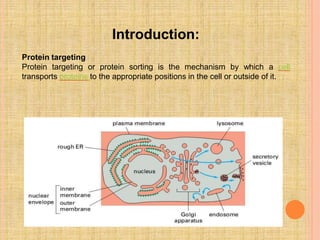
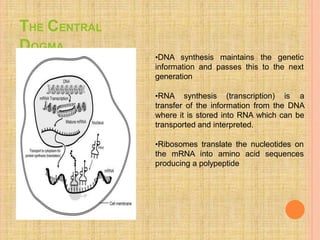
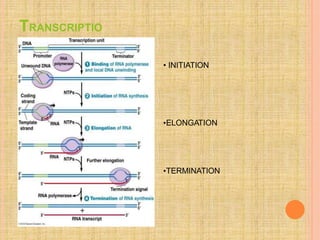
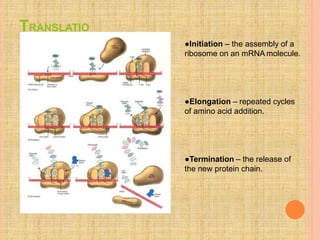
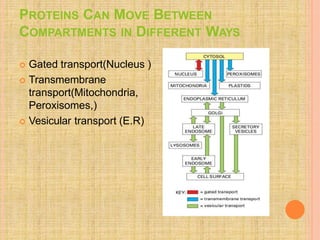
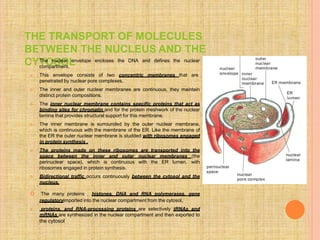
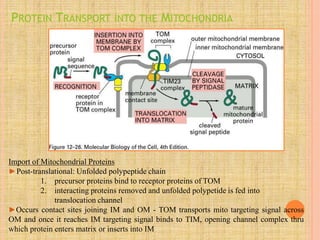
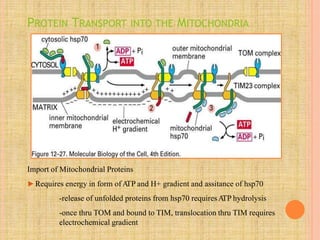
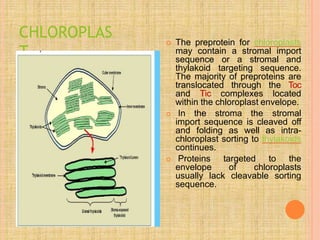
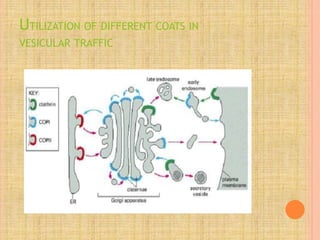
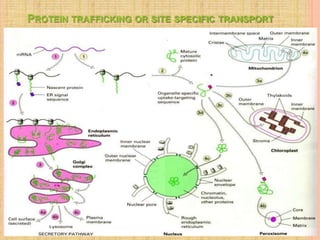
Both in prokaryotes and eukaryotes, newly synthesized proteins must be delivered to their correct subcellular locations. Secretory proteins have an N-terminal signal peptide that targets them to the endoplasmic reticulum, where they are translocated and modified. Transport vesicles then carry proteins from the ER to the Golgi complex for further modification. From the Golgi, vesicles transport proteins either to other organelles or fuse with the plasma membrane to release proteins outside the cell. Correct protein targeting and trafficking is essential for cellular function.