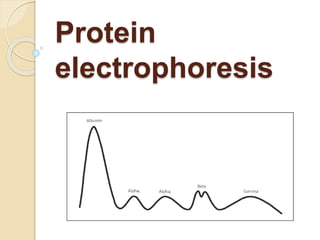
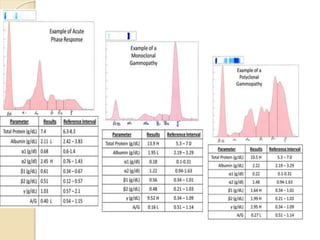
1. Protein electrophoresis separates serum proteins based on their net charge and size, allowing identification of abnormal protein levels or types.
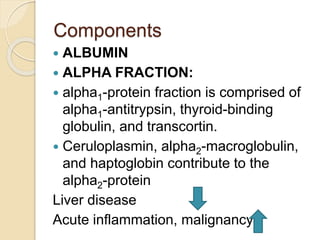
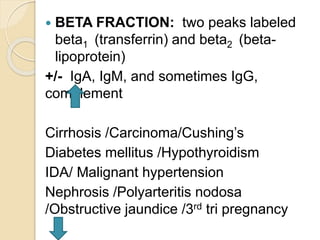
2. The major protein fractions identified include albumin, alpha, beta, and gamma globulins. Abnormal levels of these fractions can indicate conditions like myeloma, cirrhosis, or inflammation.
3. Multiple myeloma is diagnosed based on clonal bone marrow plasma cells ≥10% or other criteria like renal impairment, anemia, or bone lesions. Workup includes serum and urine protein electrophoresis and bone marrow biopsy.