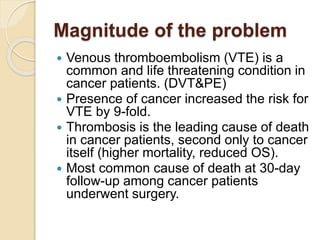
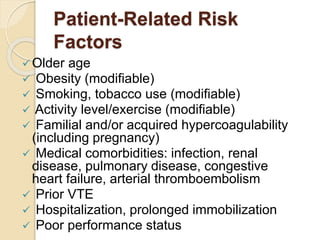
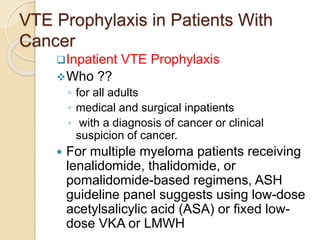
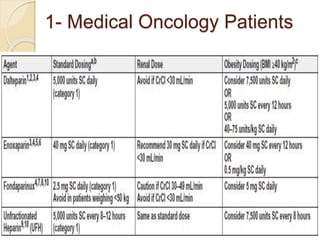
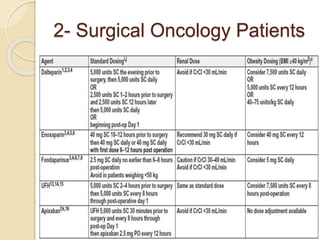
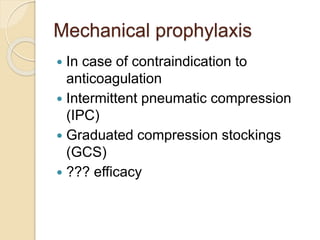
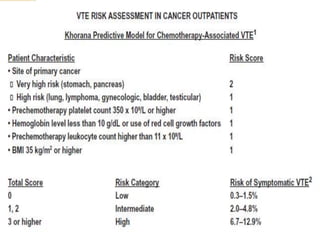
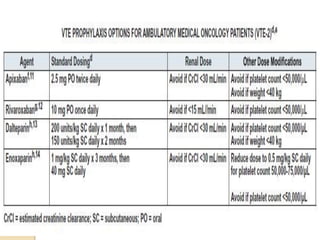
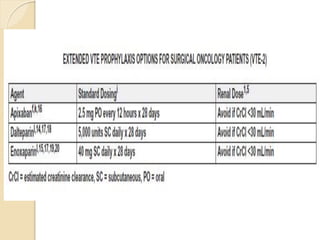
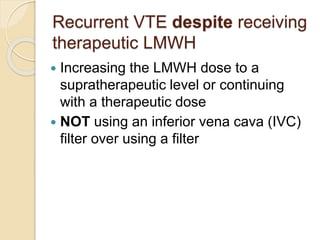
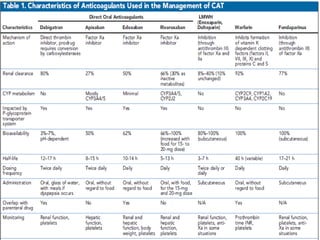
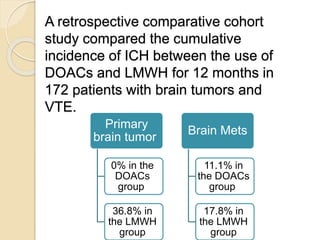
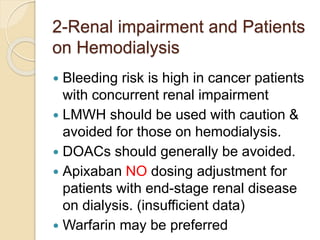
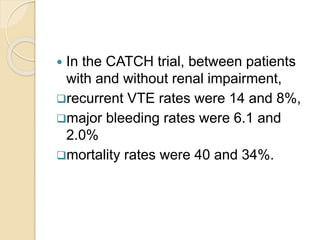
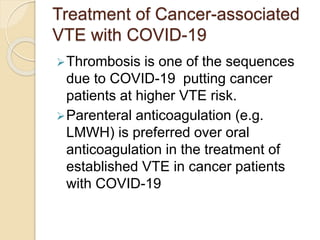
This document discusses cancer-associated thrombosis, including its high prevalence and mortality risk. Venous thromboembolism (VTE) is common in cancer patients due to patient-related, cancer-related, and treatment-related risk factors. VTE prophylaxis and treatment are important, with low molecular weight heparins and direct oral anticoagulants playing a major role. Treatment decisions require weighing risks and benefits on a case-by-case basis, with at least 6 months of anticoagulation often recommended for cancer-associated VTE. Special populations like those with renal or liver impairment require modified approaches.