The document discusses paraproteinemia, primarily focusing on multiple myeloma, characterized by monoclonal immunoglobulin or light chains in blood or urine, often leading to anemia, bone lesions, and renal complications. It outlines patient presentation, diagnosis, and various causes, as well as treatment options and the typical patient demographic. The overall management strategies are highlighted, including immunomodulatory agents and the significance of achieving clinical remission, while noting the major causes of mortality associated with the condition.













![ MM cells bind via cell-surface adhesion molecules to bone marrow stromal cells
(BMSCs) and extracellular matrix (ECM), which triggers MM cell growth, survival,
drug resistance, and migration into the bone marrow.
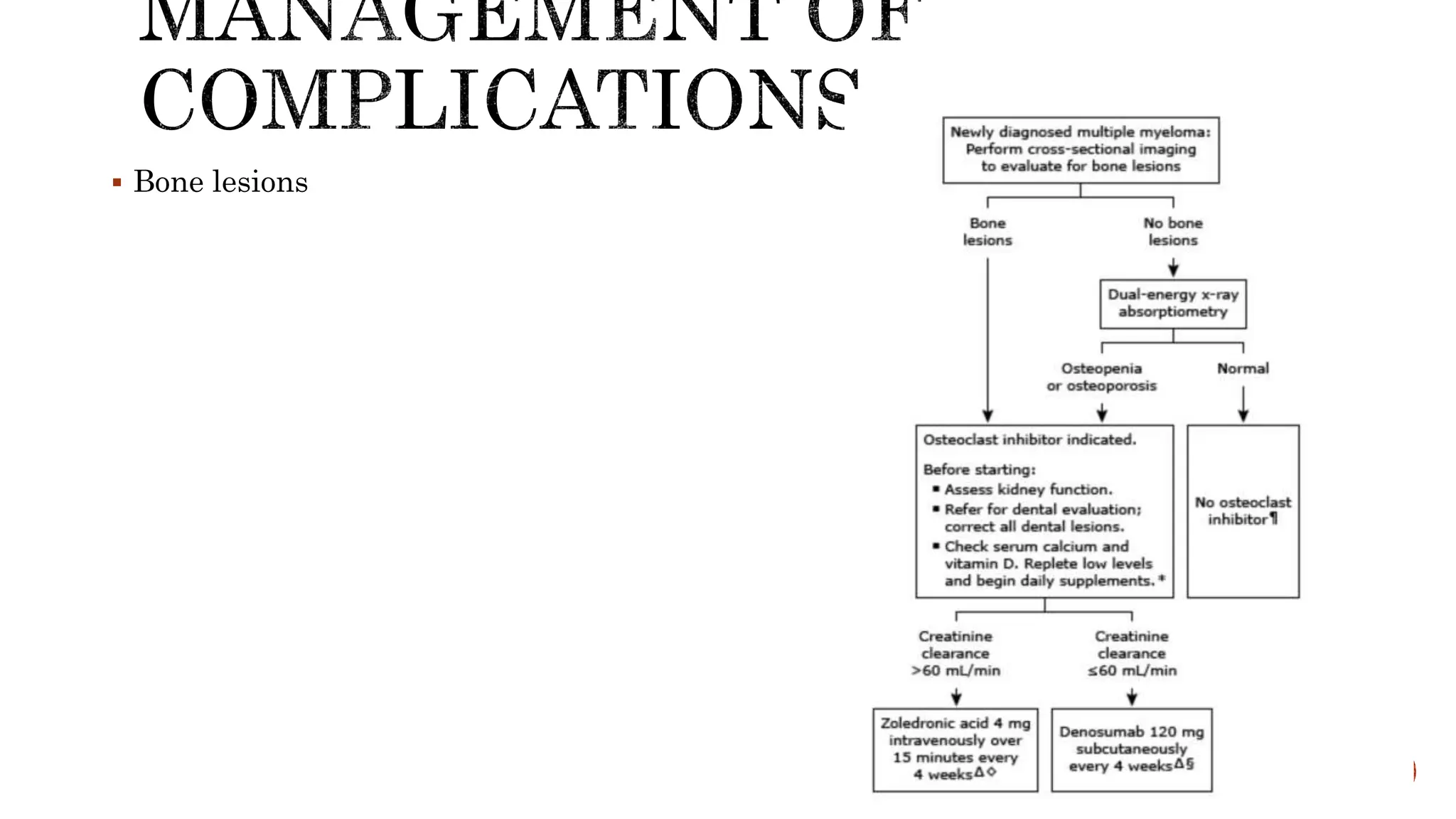
Bone pain: Most common symptom. The bone lesions of myeloma are caused by the
proliferation of tumor cells, activation of osteoclasts that destroy bone,and
suppression of osteoblasts that form new bone. The increased osteoclast activity is
mediated by osteoclast activating factors (OAFs) produced by the myeloma cells
(mediated by several cytokines, includingIL-1, lymphotoxin, vascular endothelial
growth factor [VEGF],receptor activator of nuclear factor-κB [RANK] ligand,
macrophageinhibitory factor [MIP]-1α, and tumor necrosis factor [TNF]). The bone
lesions are lytic in nature. Localized bone lesions may cause the collapse of vertebrae, leading
to spinal cord compression.](https://image.slidesharecdn.com/multiplemyeloma-240502160934-3f2ac5c4/75/multiple-myeloma-features-and-findings-including-treatment-14-2048.jpg)

![ Renal Failure: Hypercalcemia is the most common cause of renal failure. Glomerular deposits of
amyloid, hyperuricemia, recurrent infections, frequent use of nonsteroidal anti-inflammatory agents
for pain control, use of iodinated contrast dye for imaging, bisphosphonate use, and occasional
infiltration of the kidney by myeloma cells all may contribute to renal dysfunction.
Tubular damage associated with the excretion of light chains is almost always present. Normally,
light chains are filtered, reabsorbed in the tubules, and catabolized. With the increase in the amount
of light chains presented to the tubule, the tubular cells become overloaded with these proteins, and
tubular damage results either directly from light chain toxic effects or indirectly from the release of
intracellular lysosomal enzymes. The earliest manifestation of this tubular damage is the adult
Fanconi’s syndrome (a type 2 proximal renal tubular acidosis), with loss of glucose and amino acids,
as well as defects in the ability of the kidney to acidify and concentrate the urine. The proteinuria is
not accompanied by hypertension, and the protein is nearly all light chains. Generally, very little
albumin is in the urine because glomerular function is usually normal. Patients with myeloma also
have a decreased anion gap [i.e., Na+ – (Cl− + HCO3−)] because the M component is cationic,
resulting in retention of chloride. Renal dysfunction due to light chain deposition disease, light chain
cast nephropathy, and amyloidosis is partially reversible with effective therapy](https://image.slidesharecdn.com/multiplemyeloma-240502160934-3f2ac5c4/75/multiple-myeloma-features-and-findings-including-treatment-16-2048.jpg)