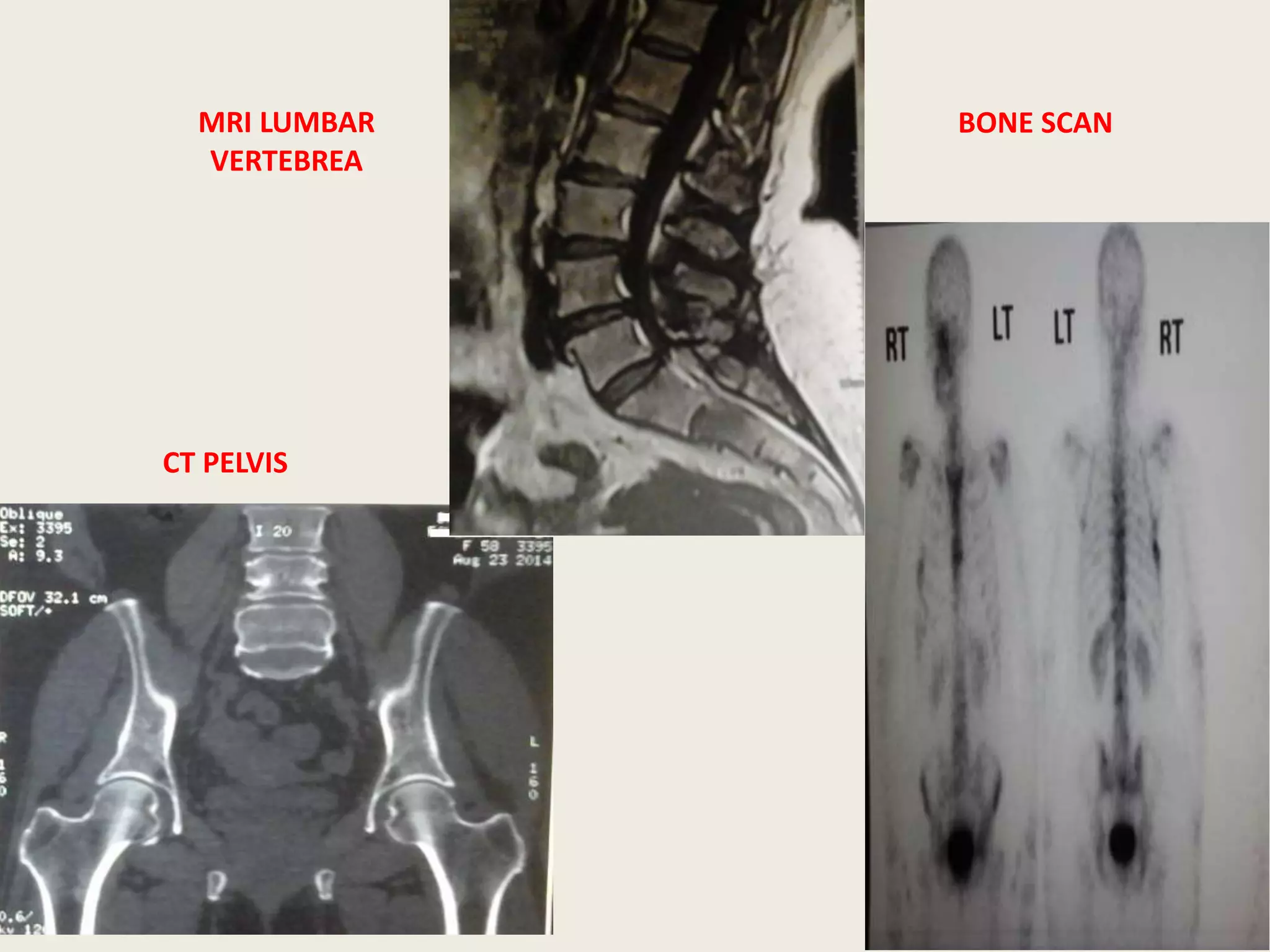
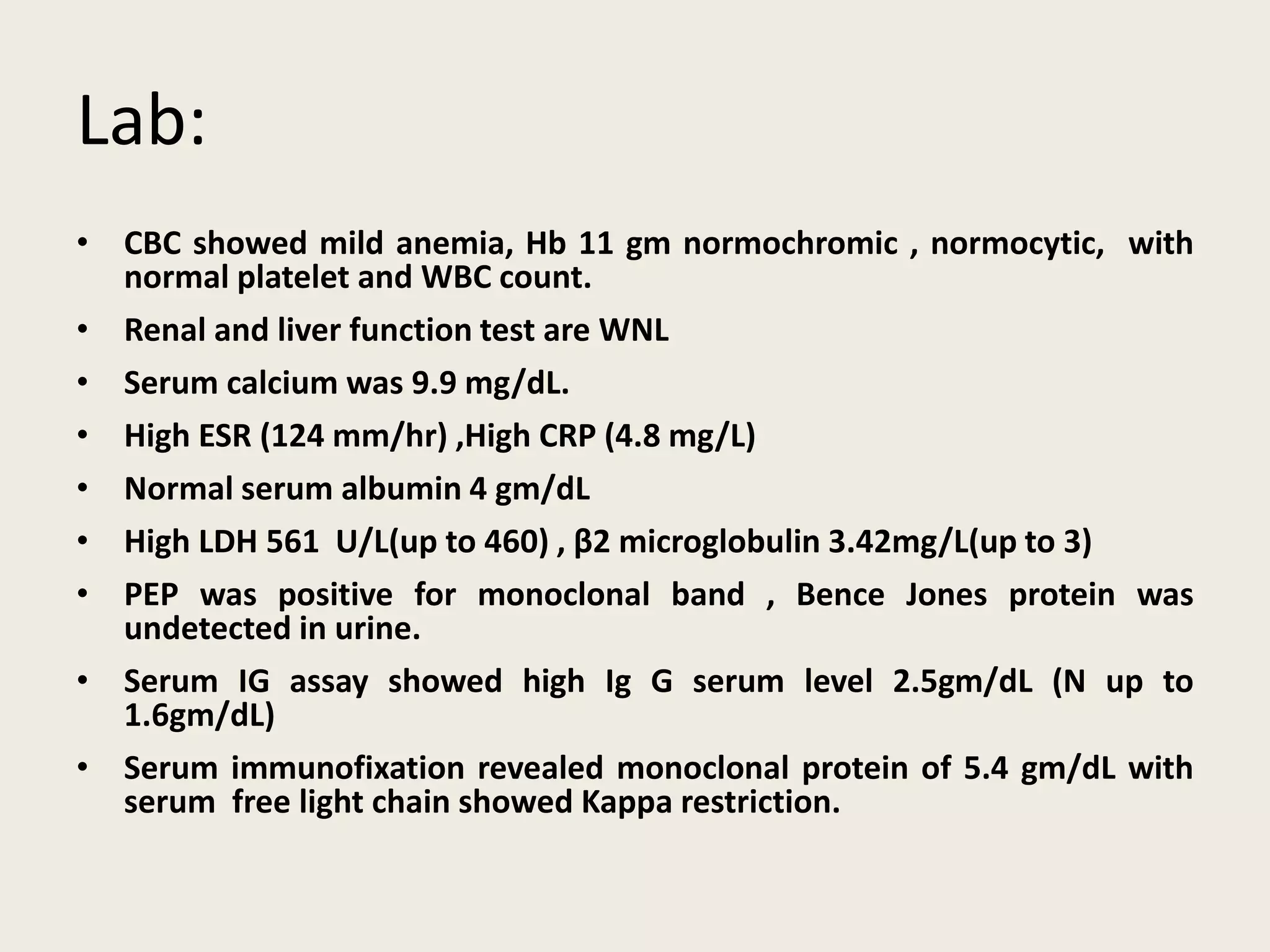
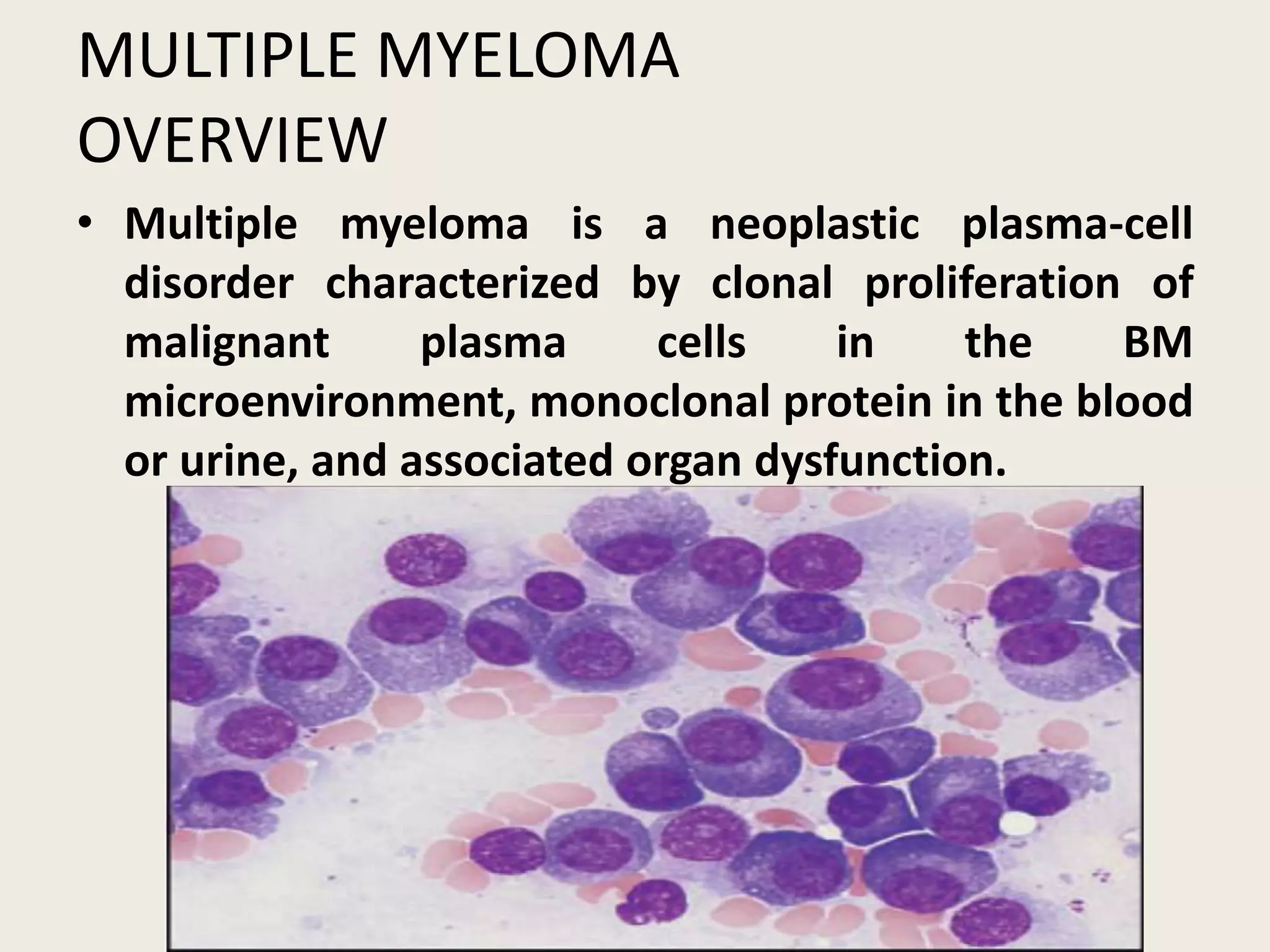
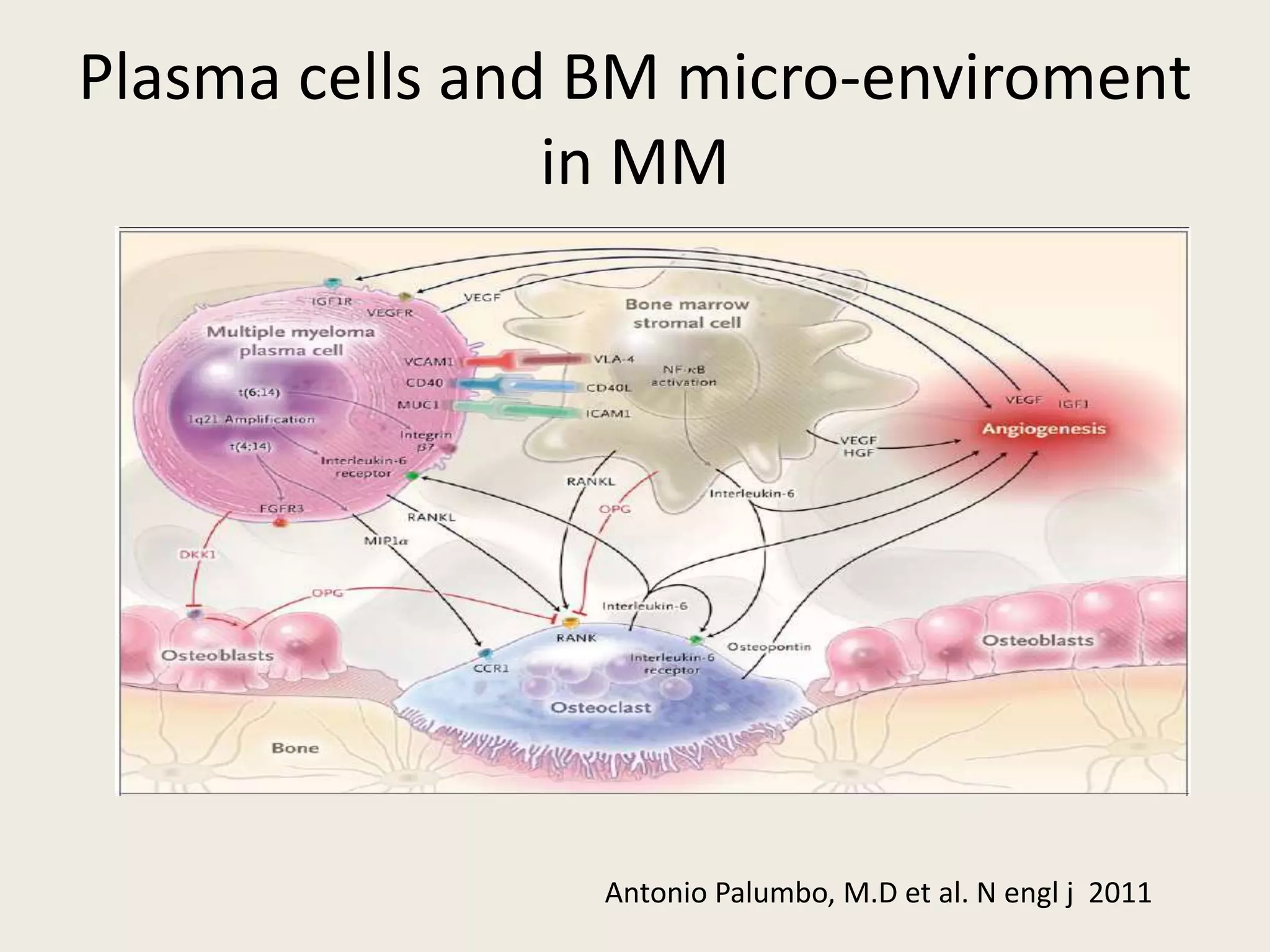
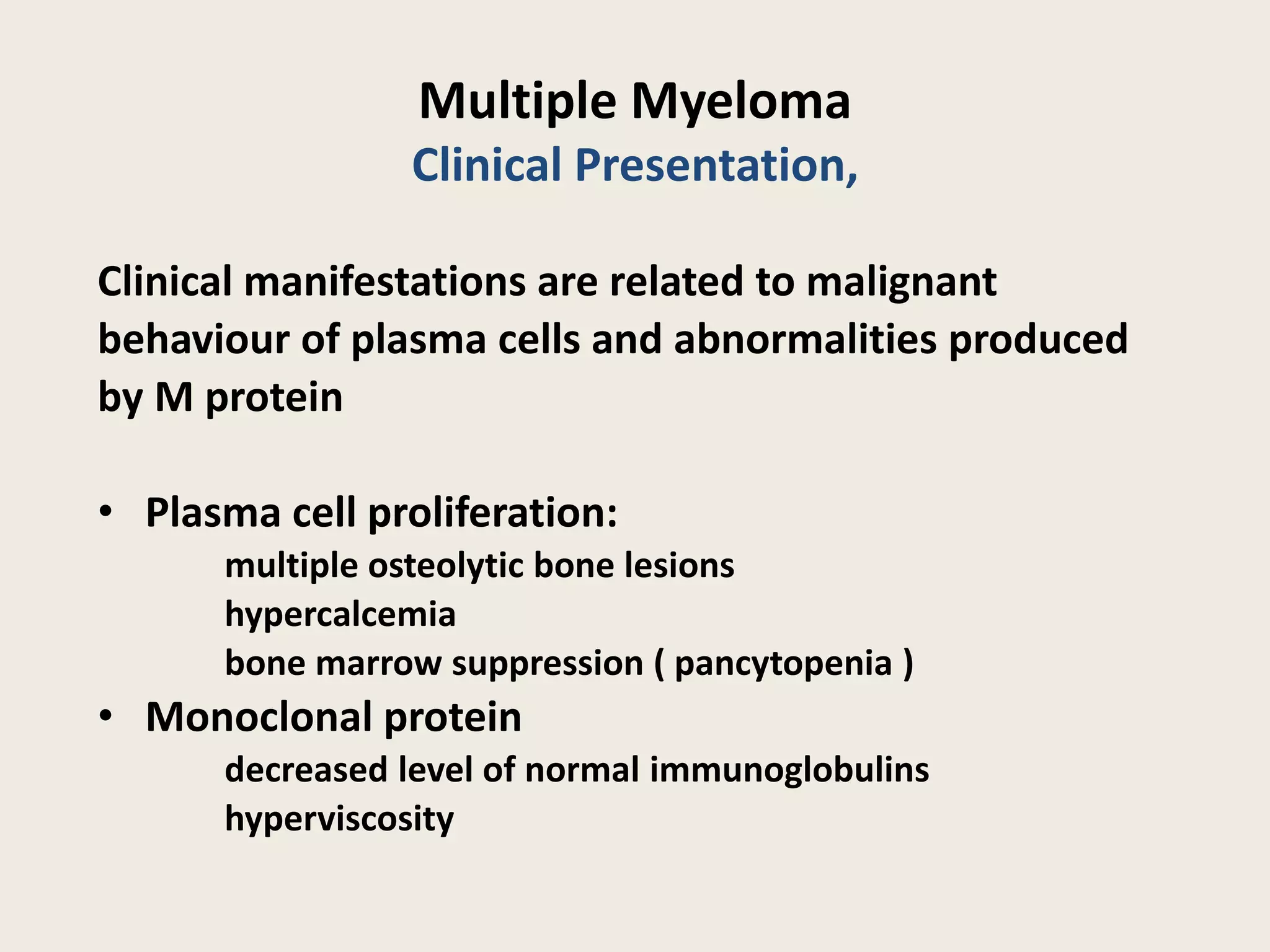
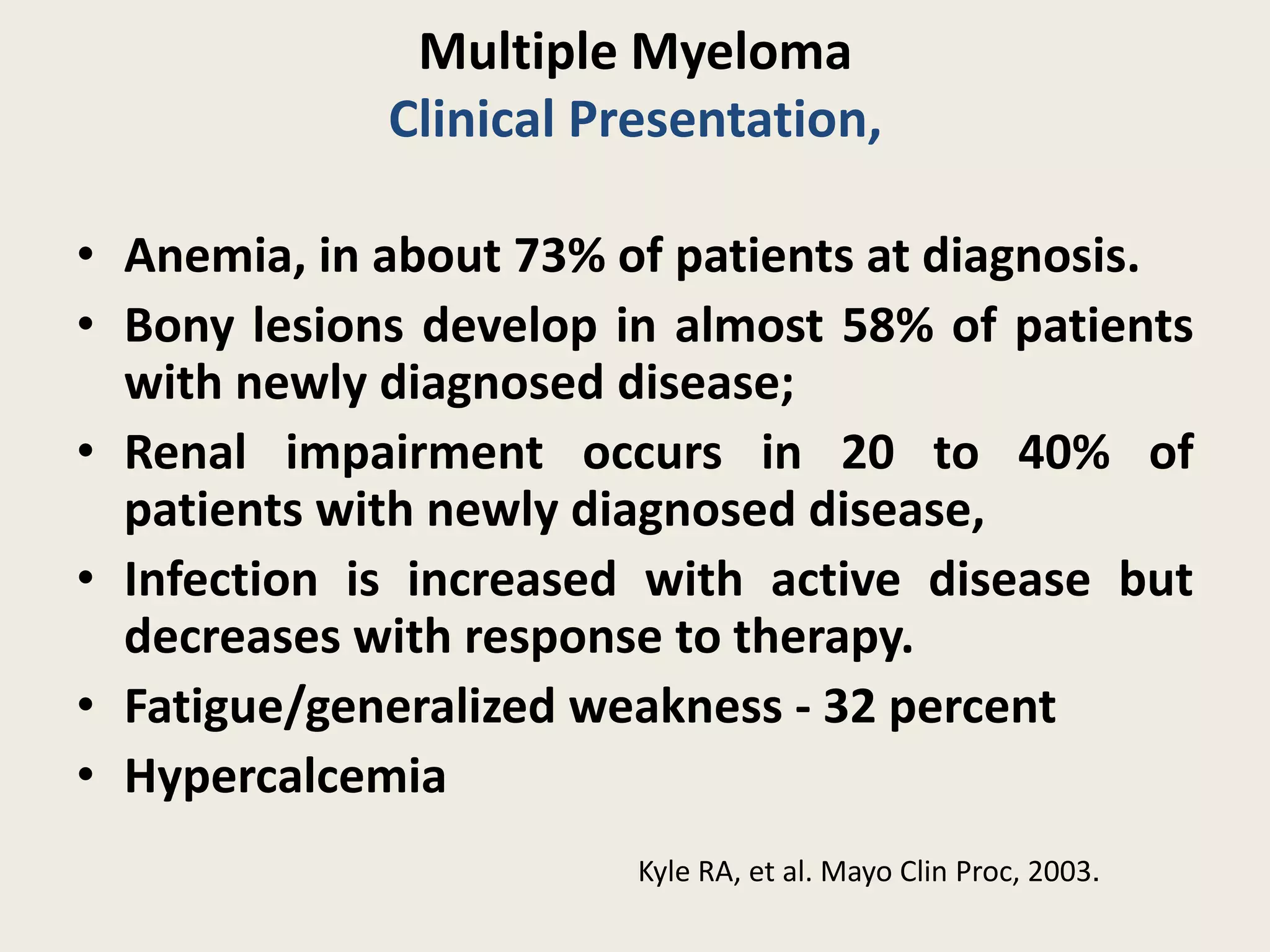
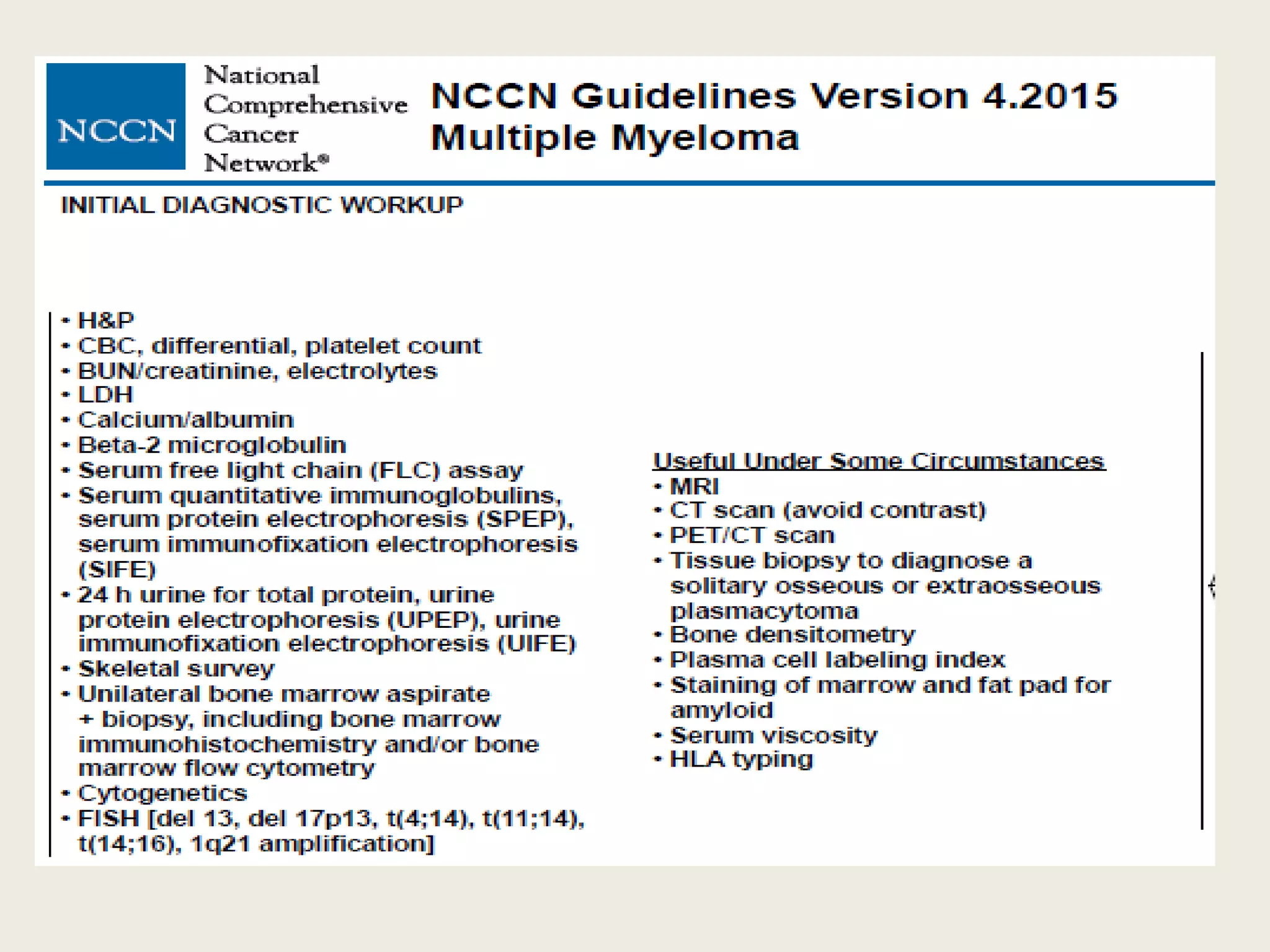
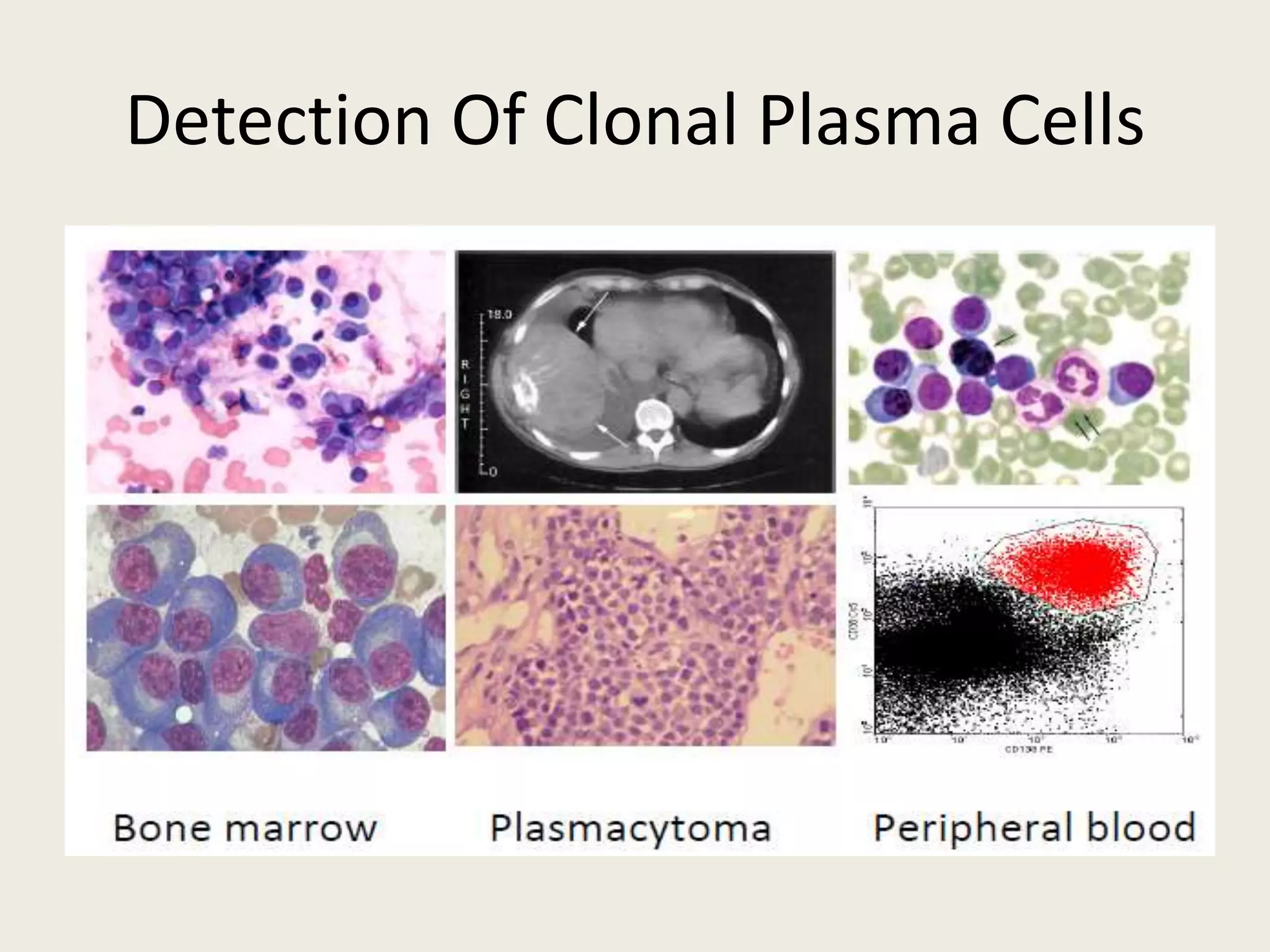
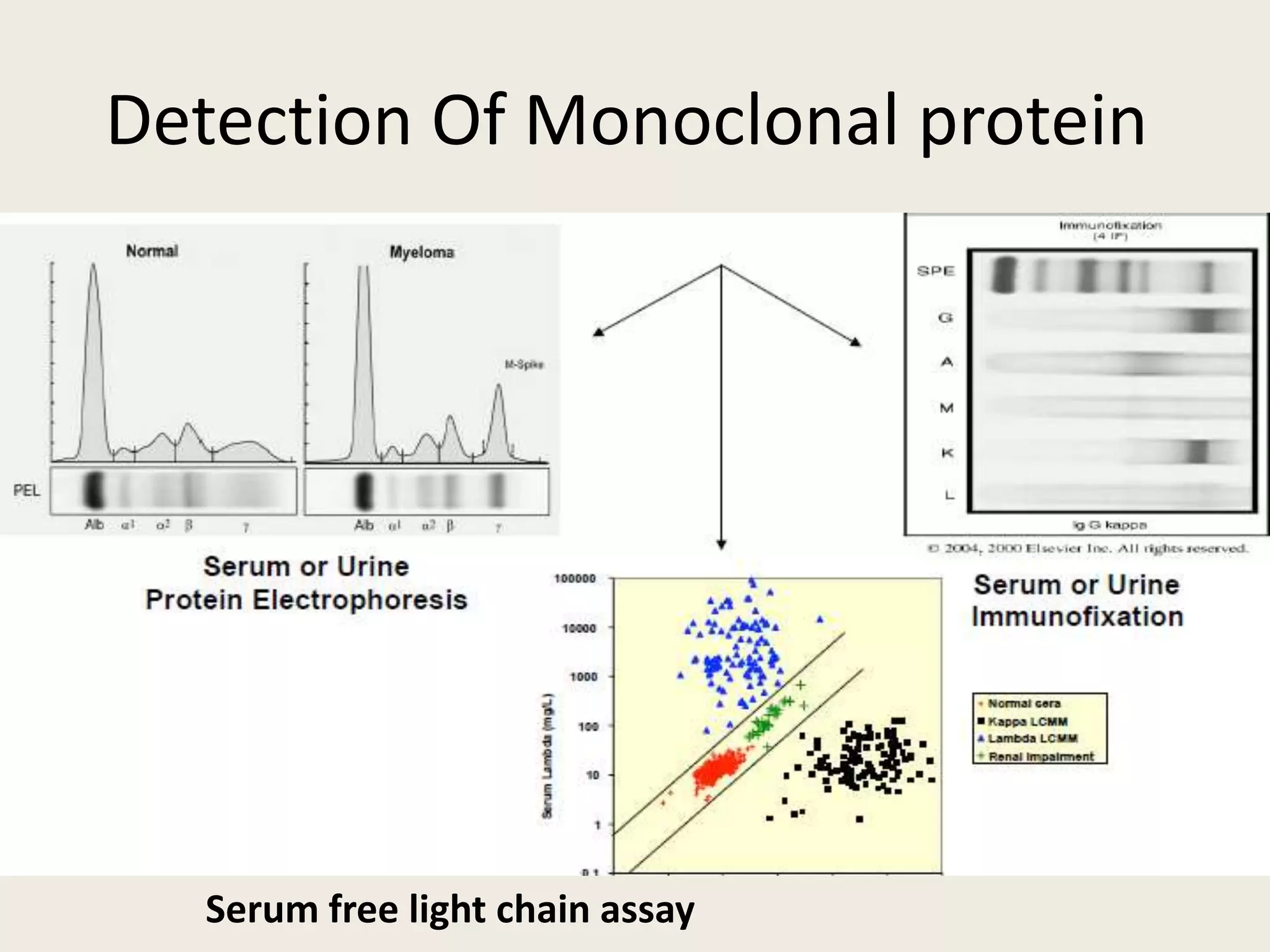
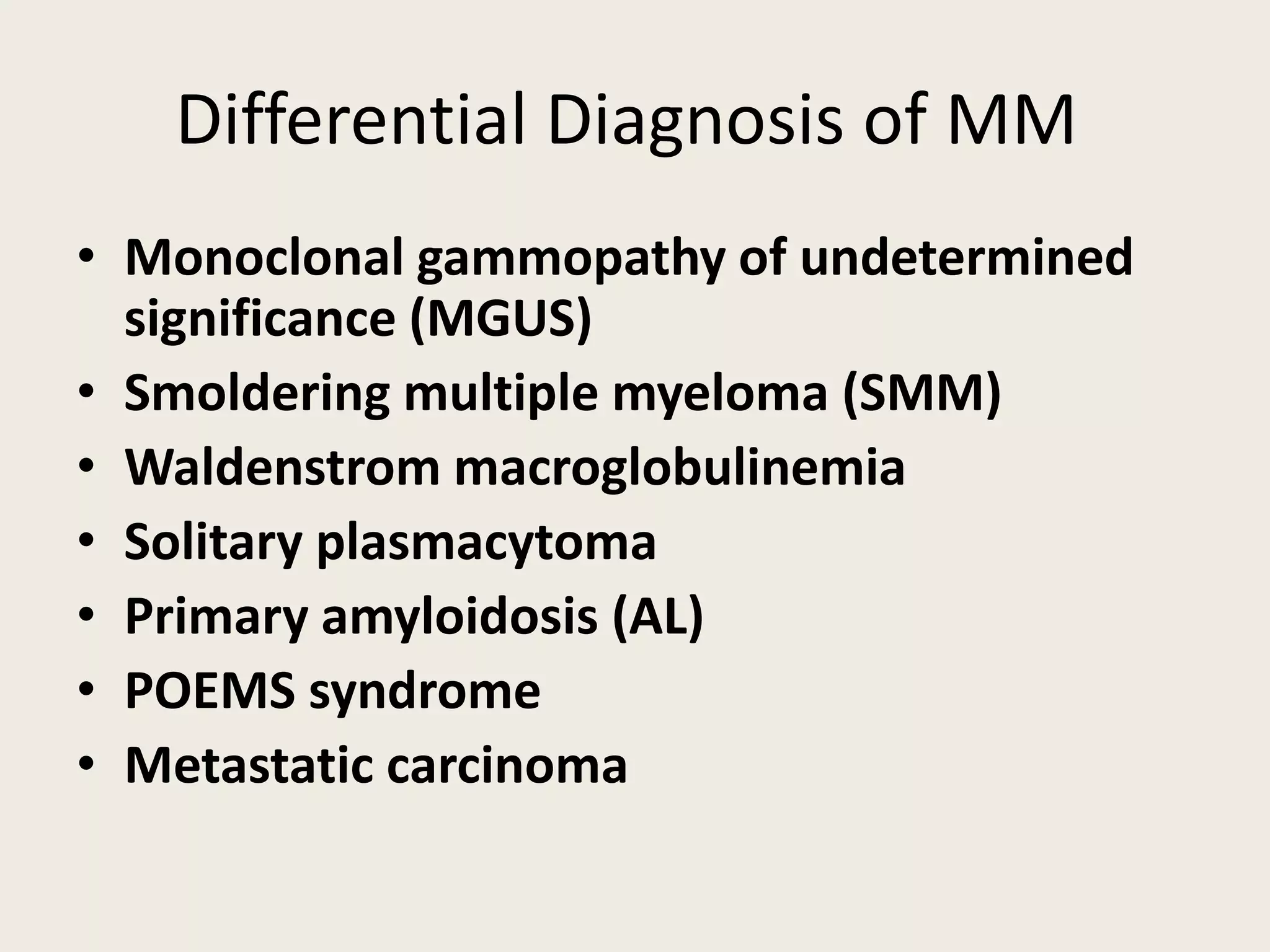
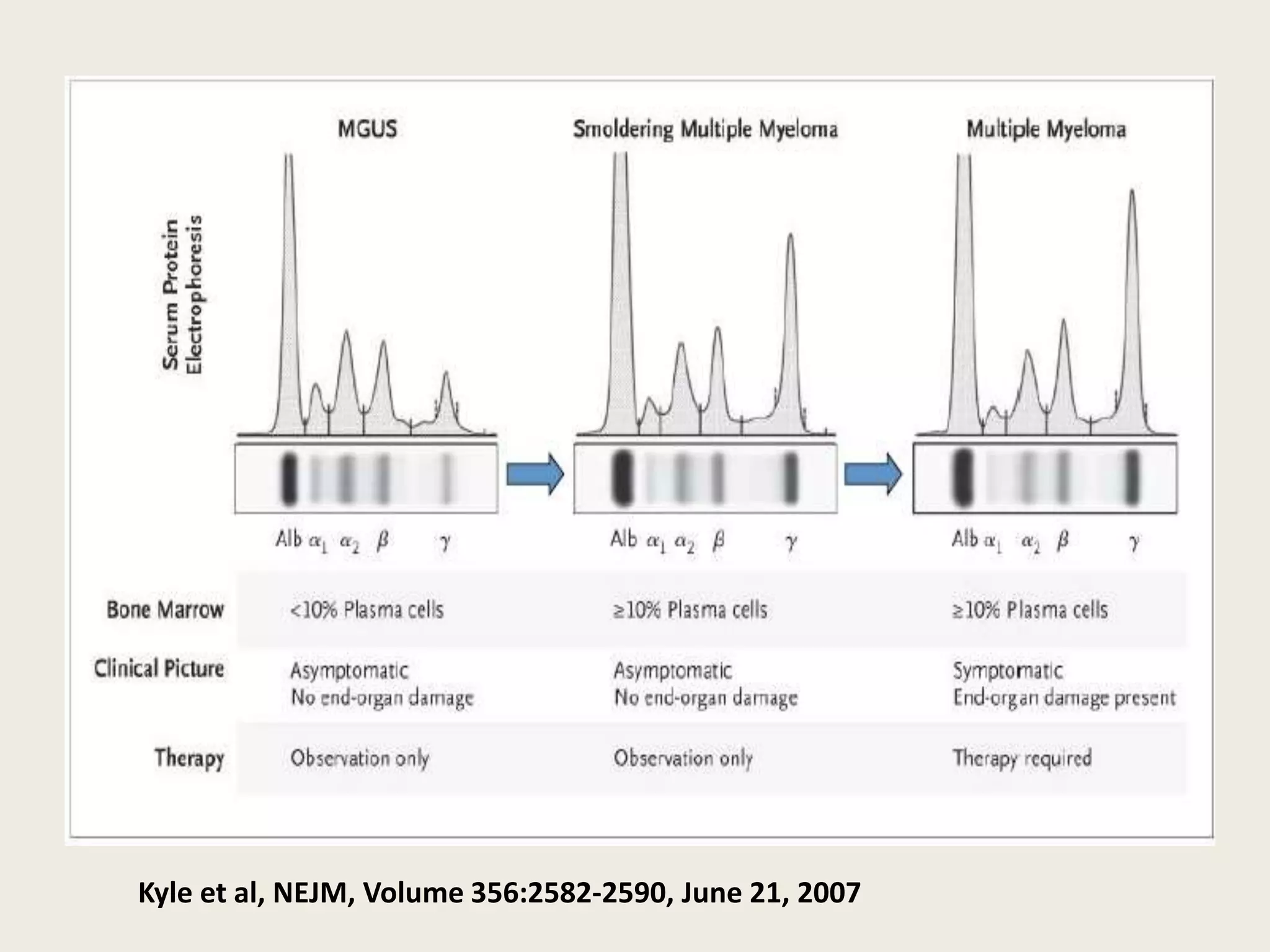
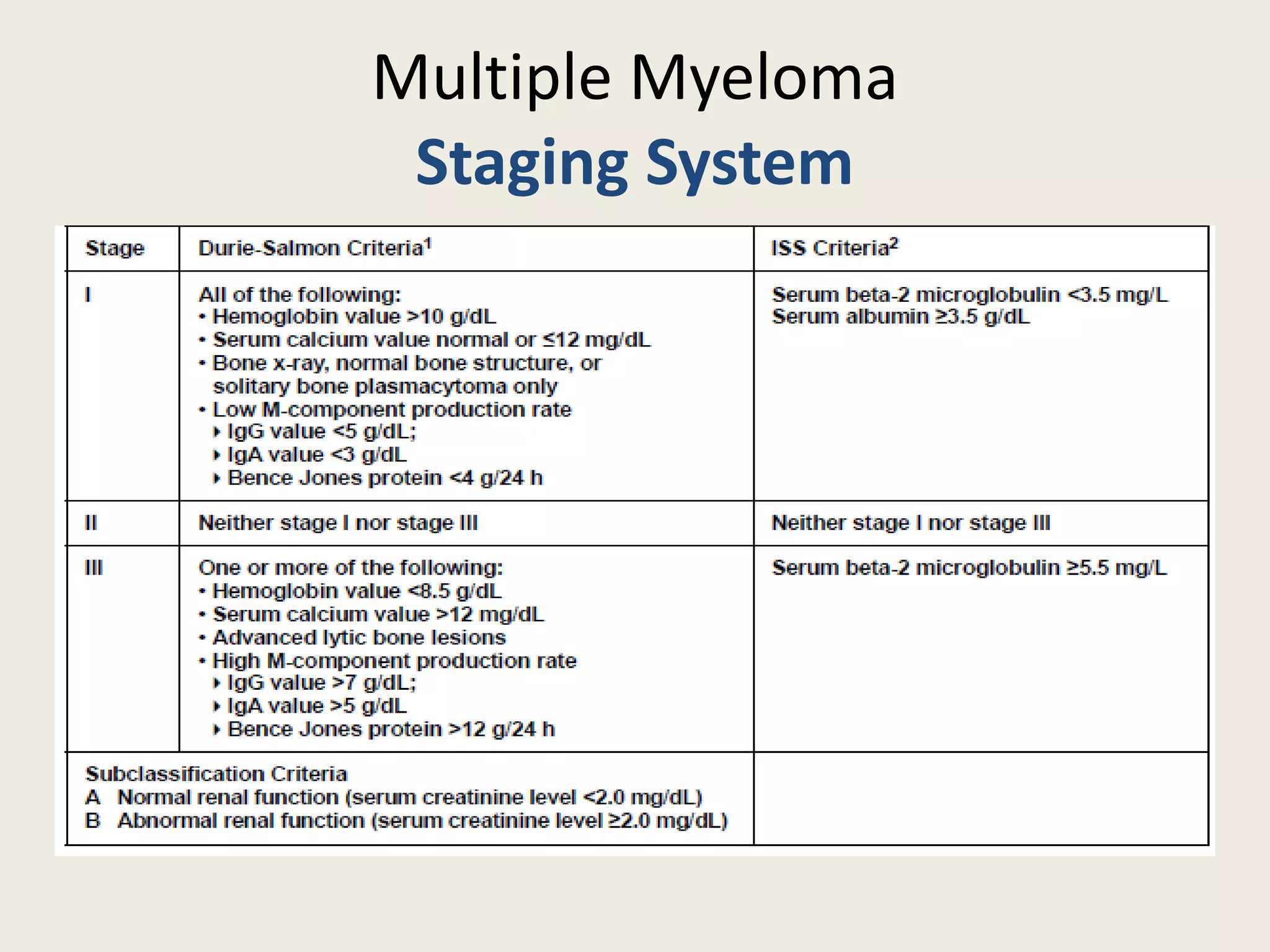
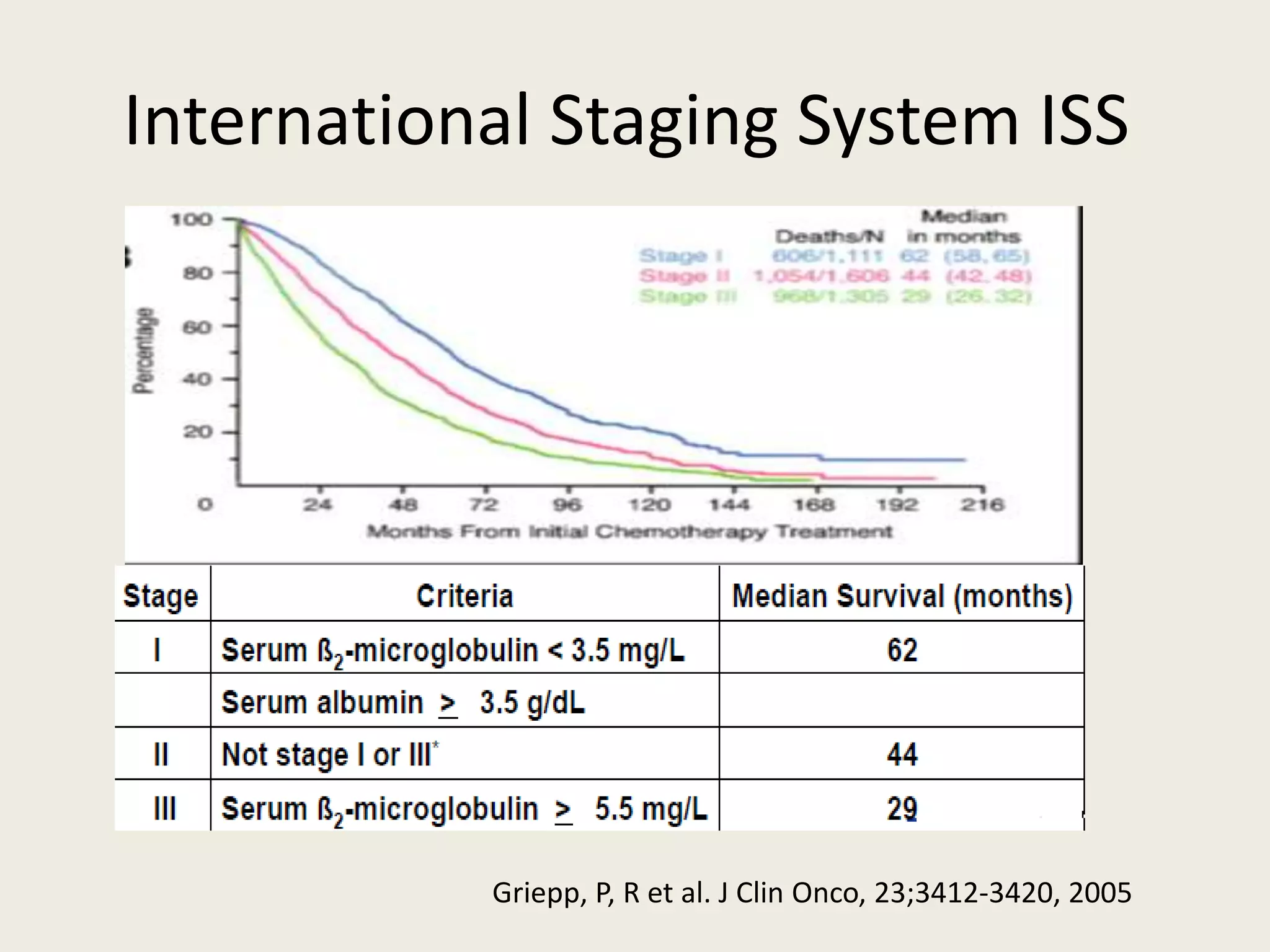
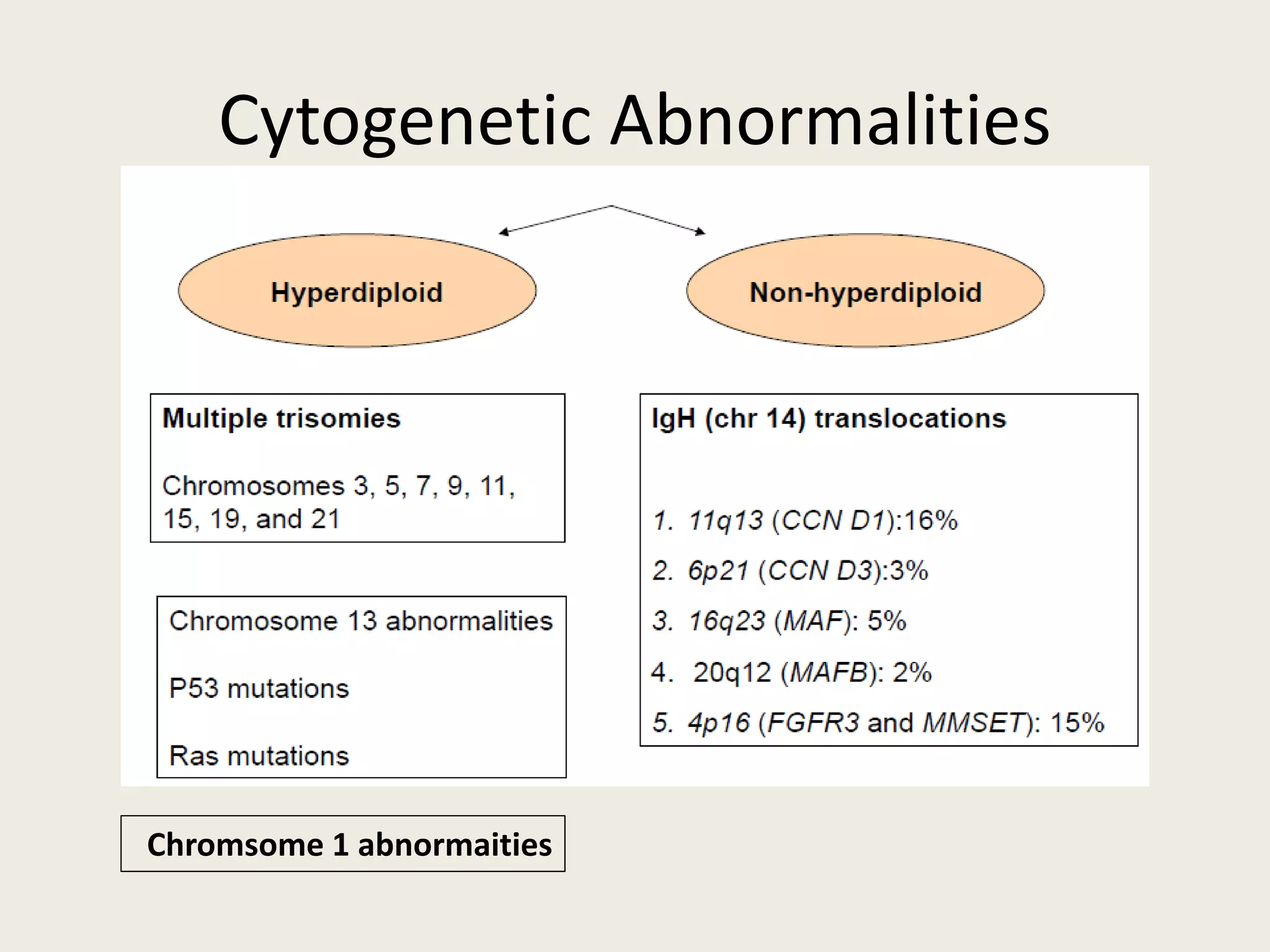
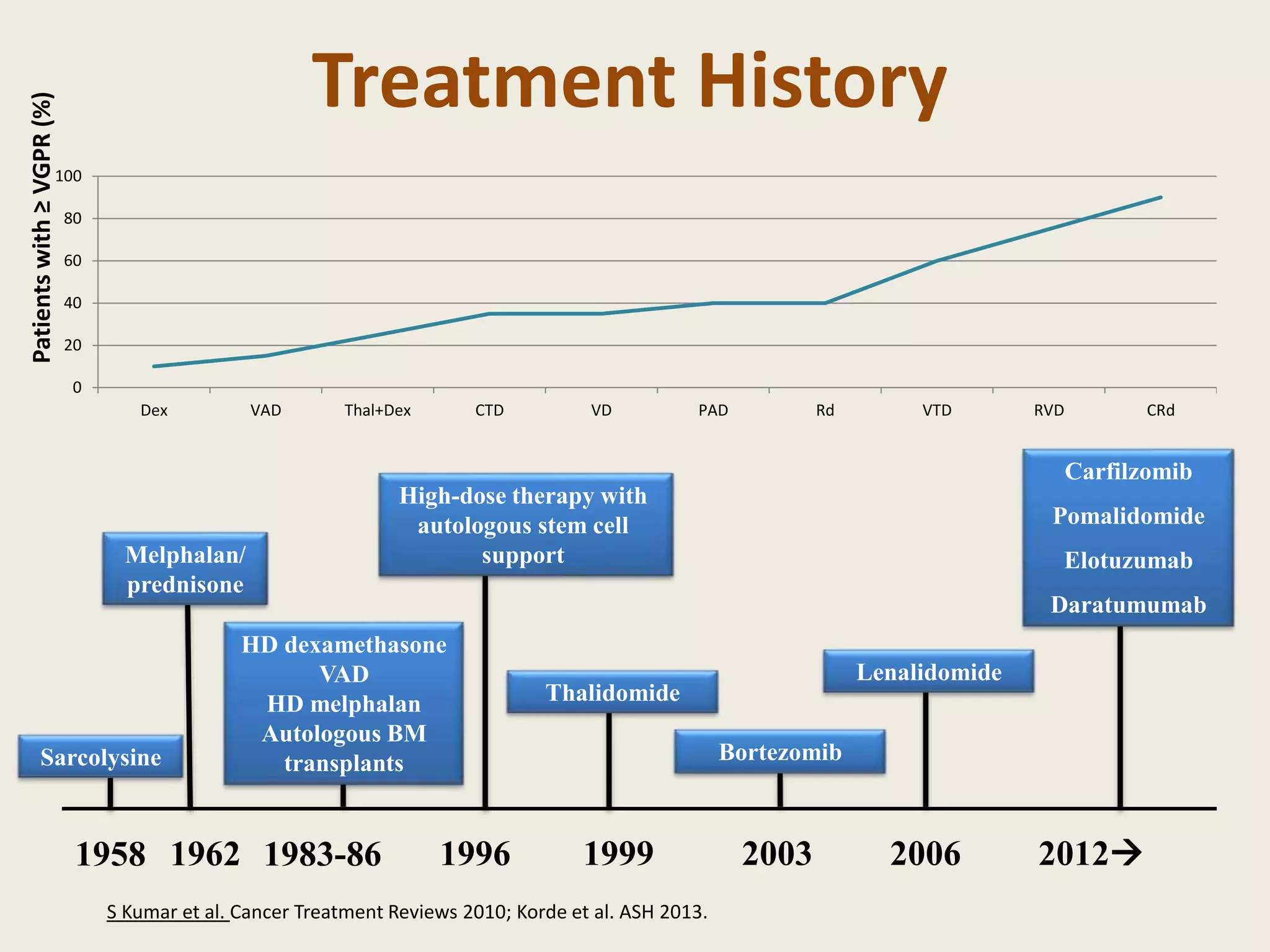
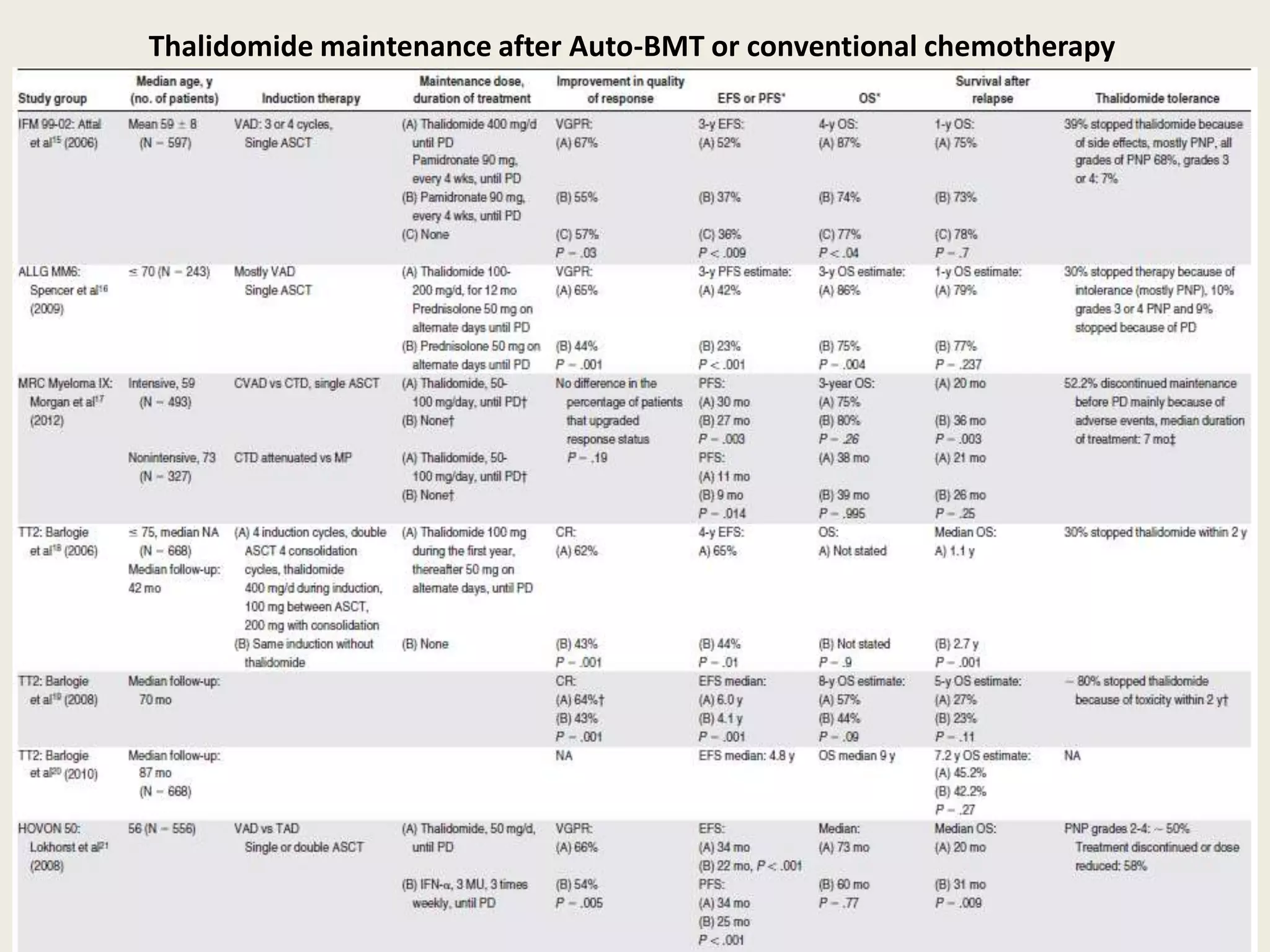
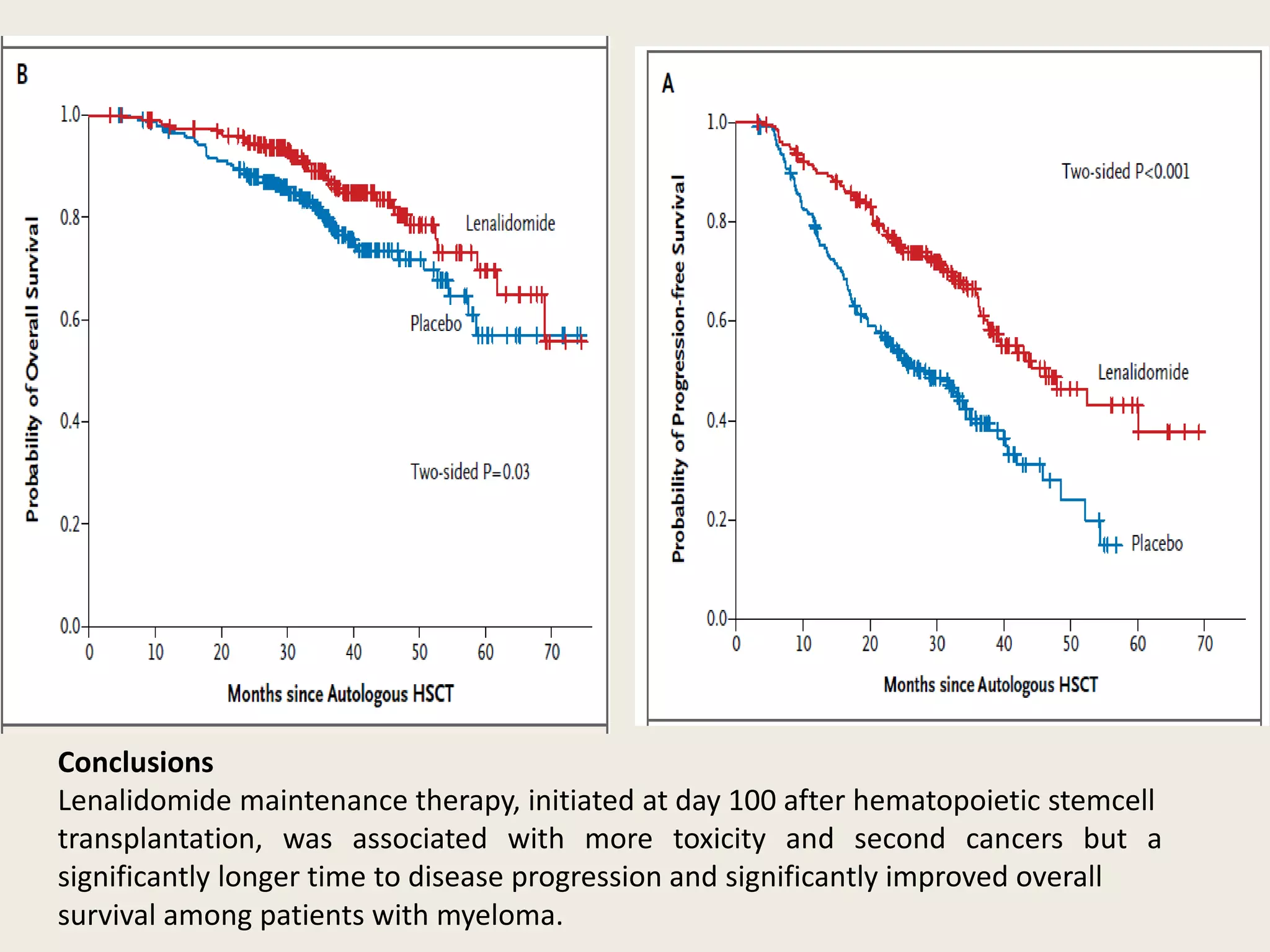
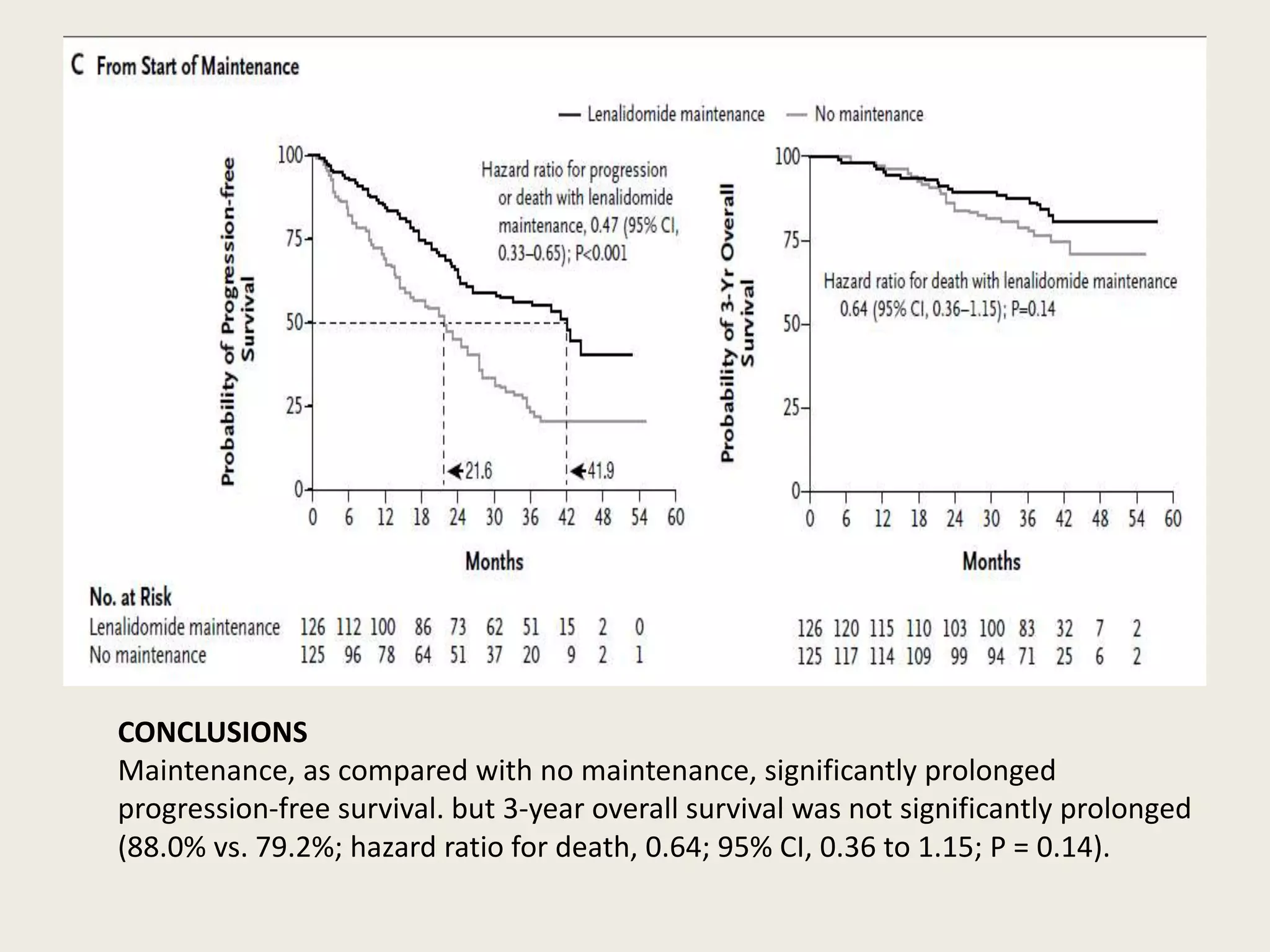
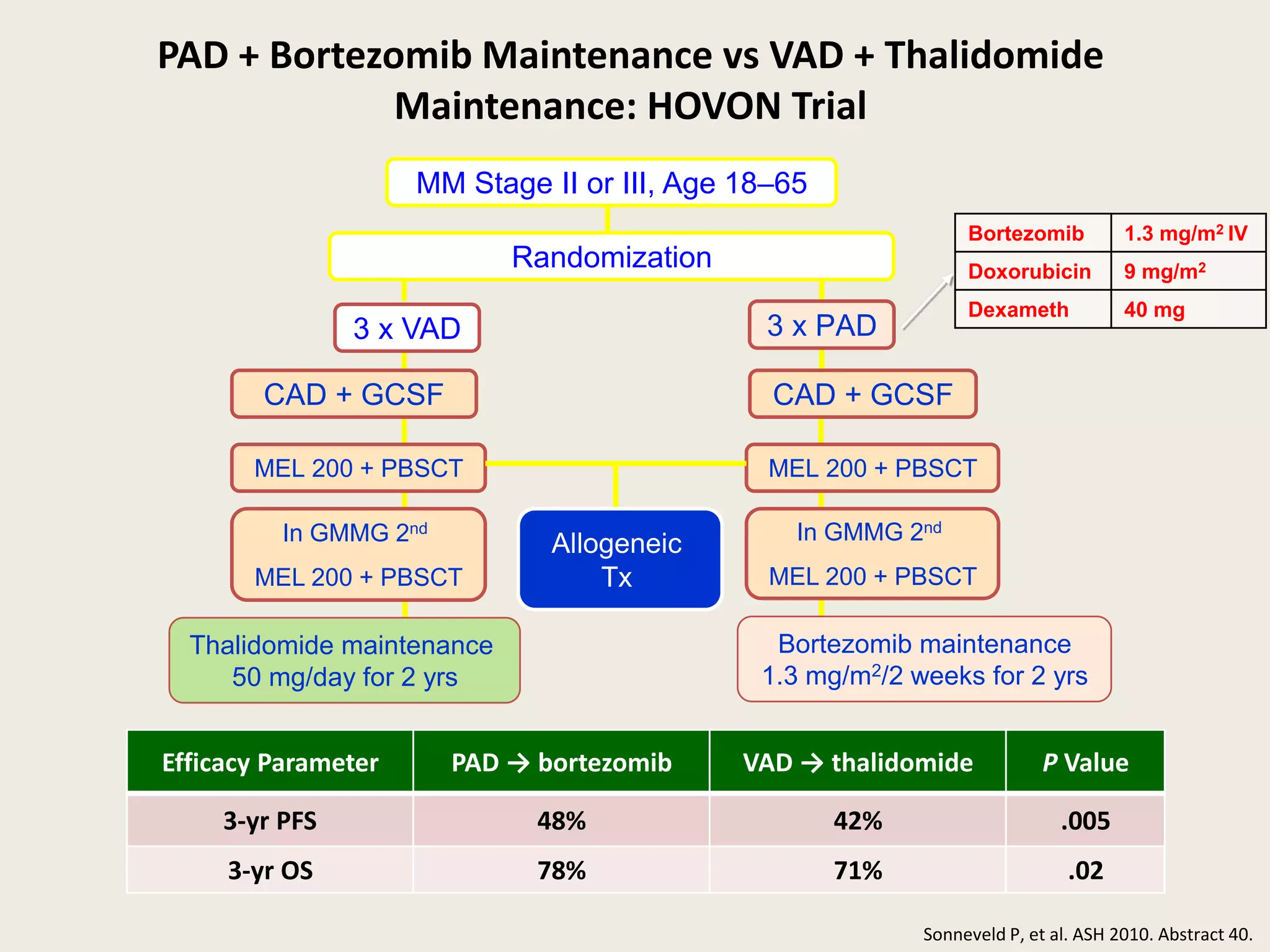
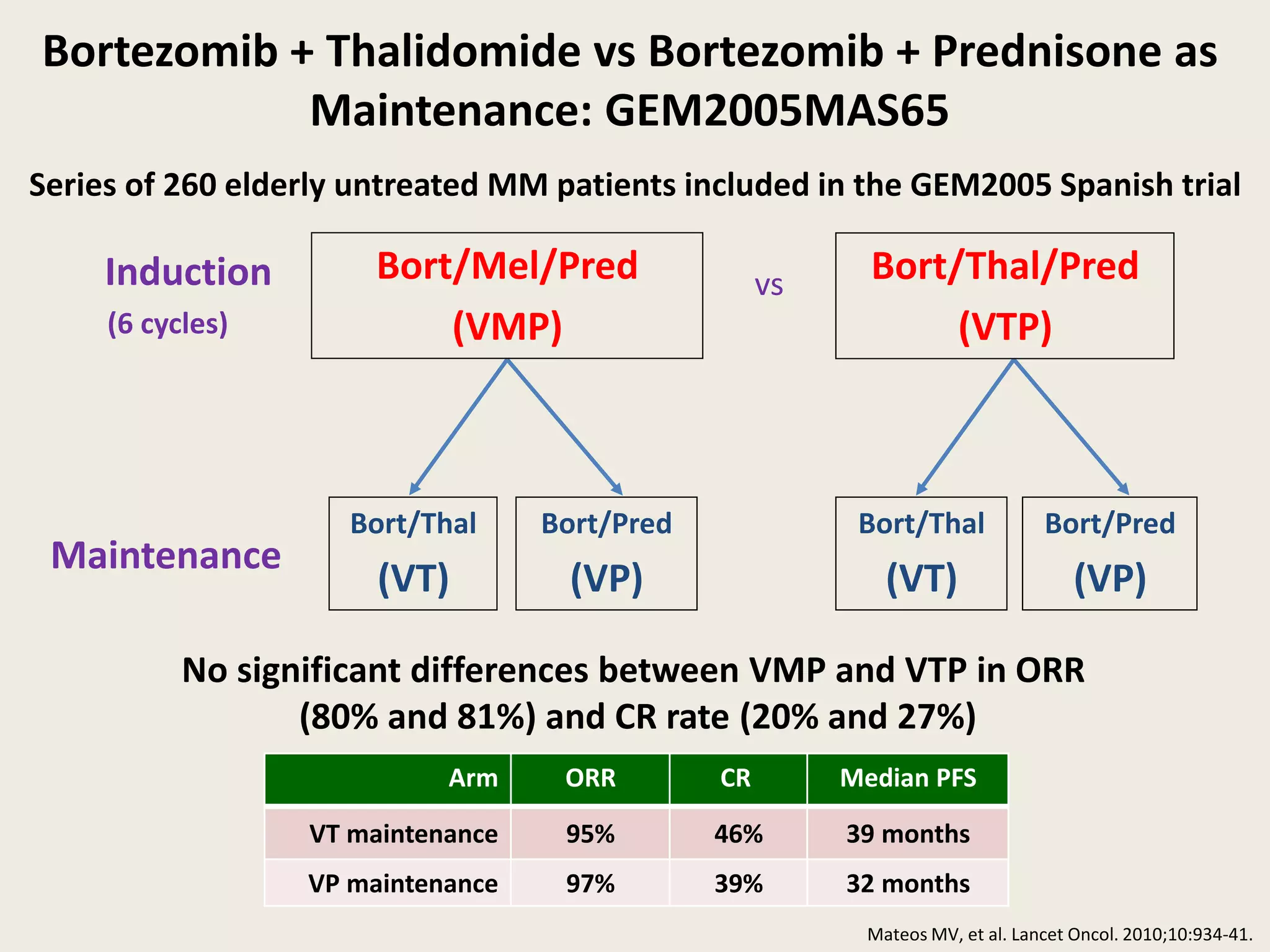
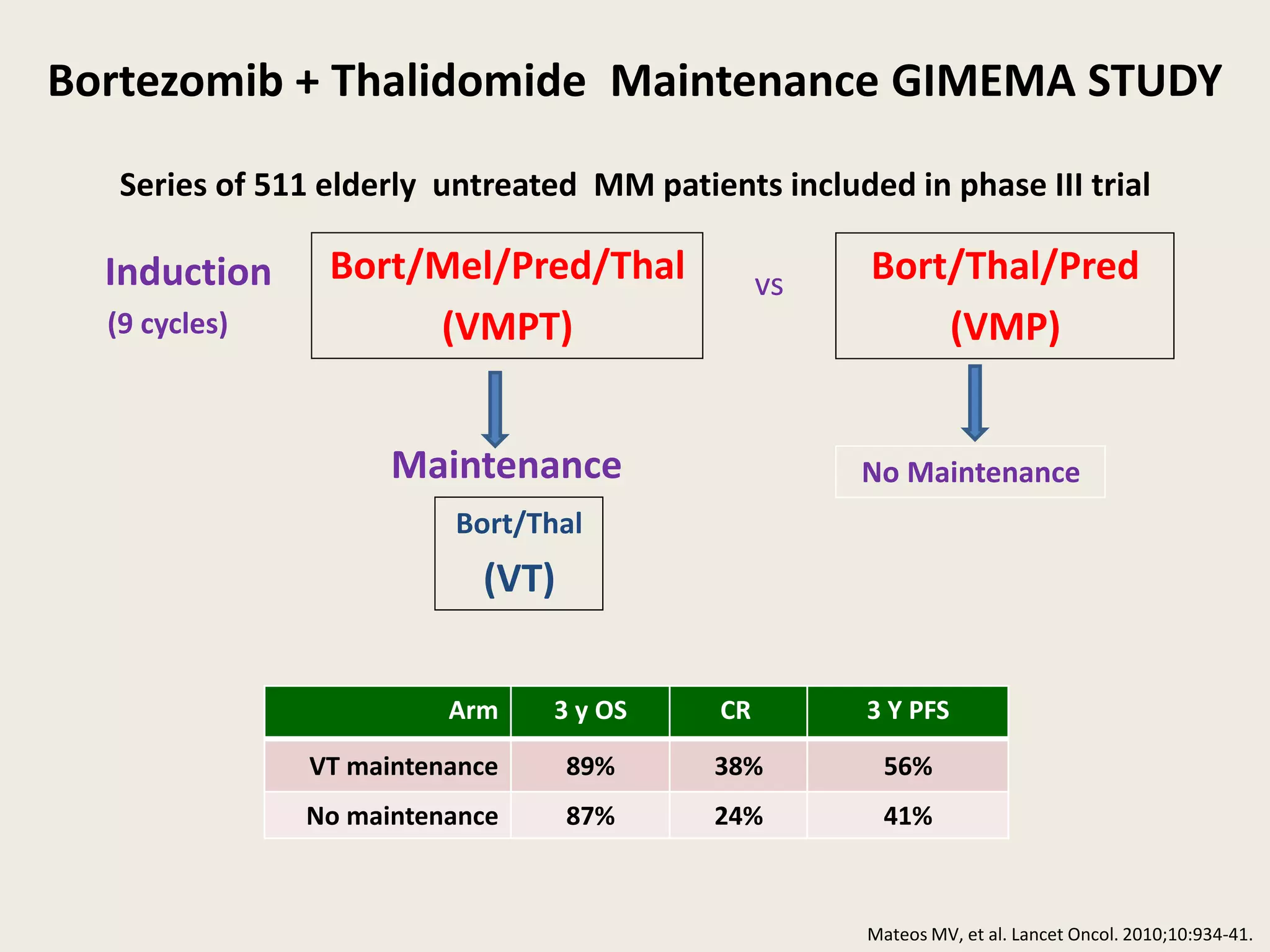
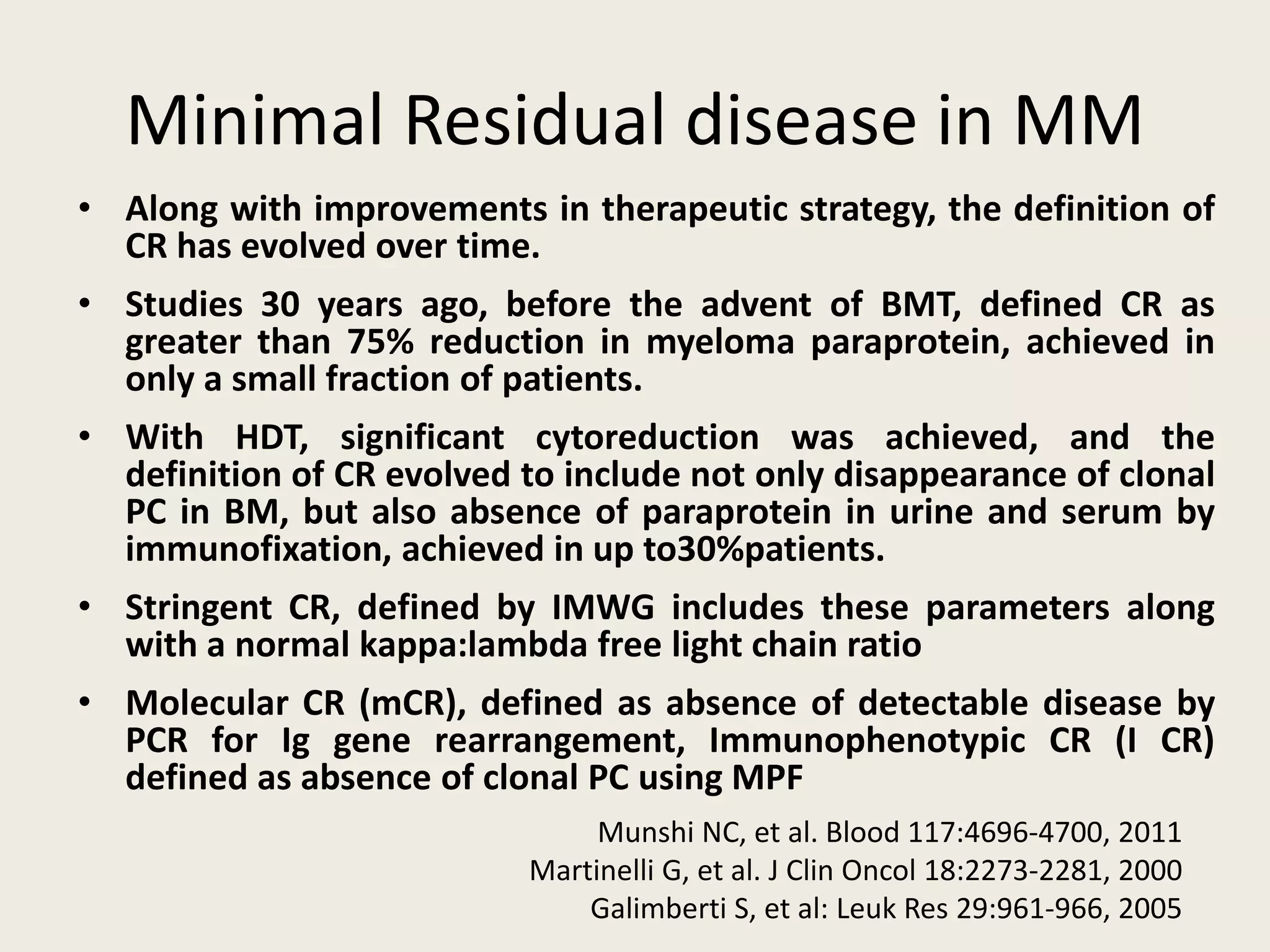
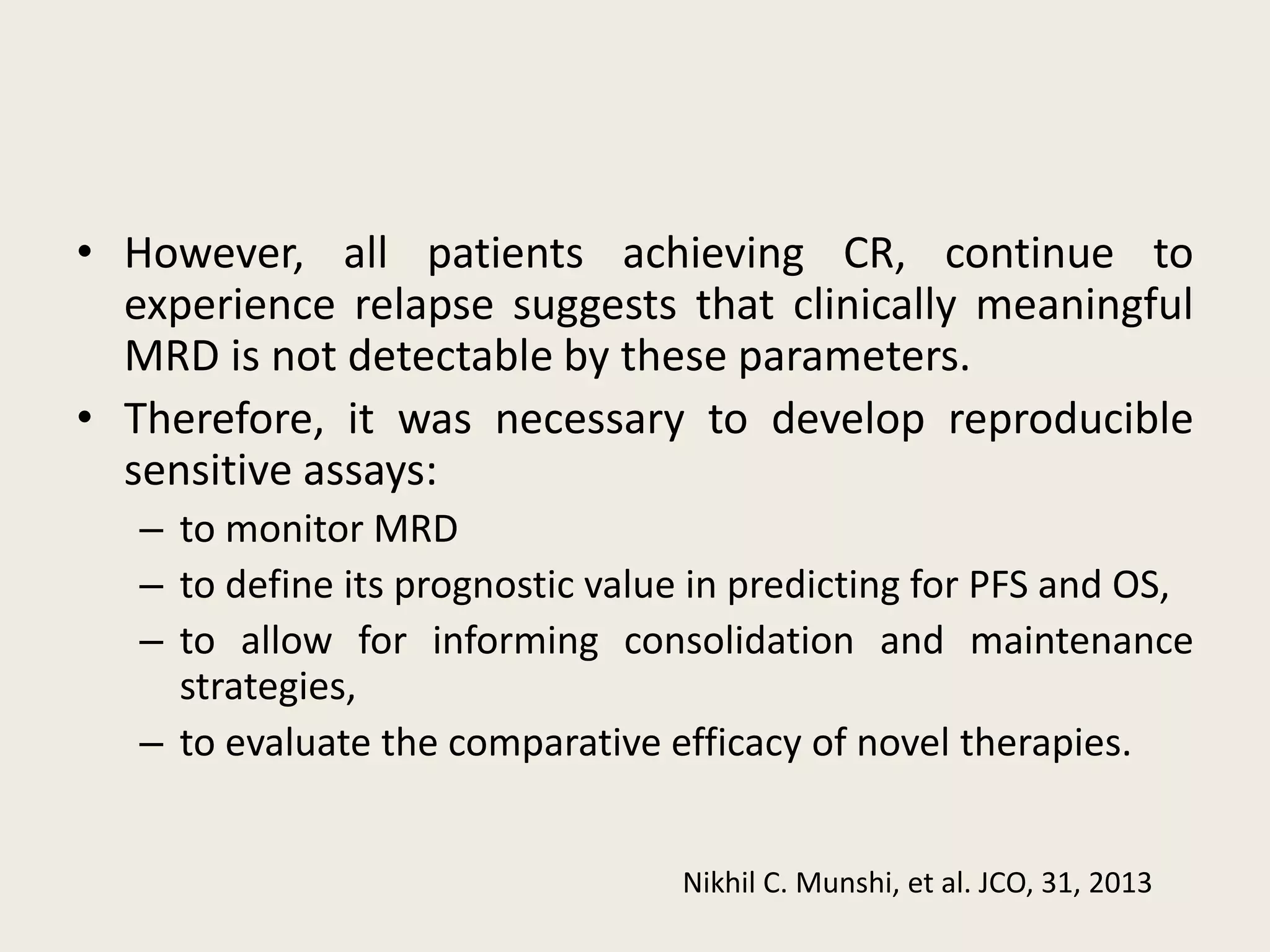
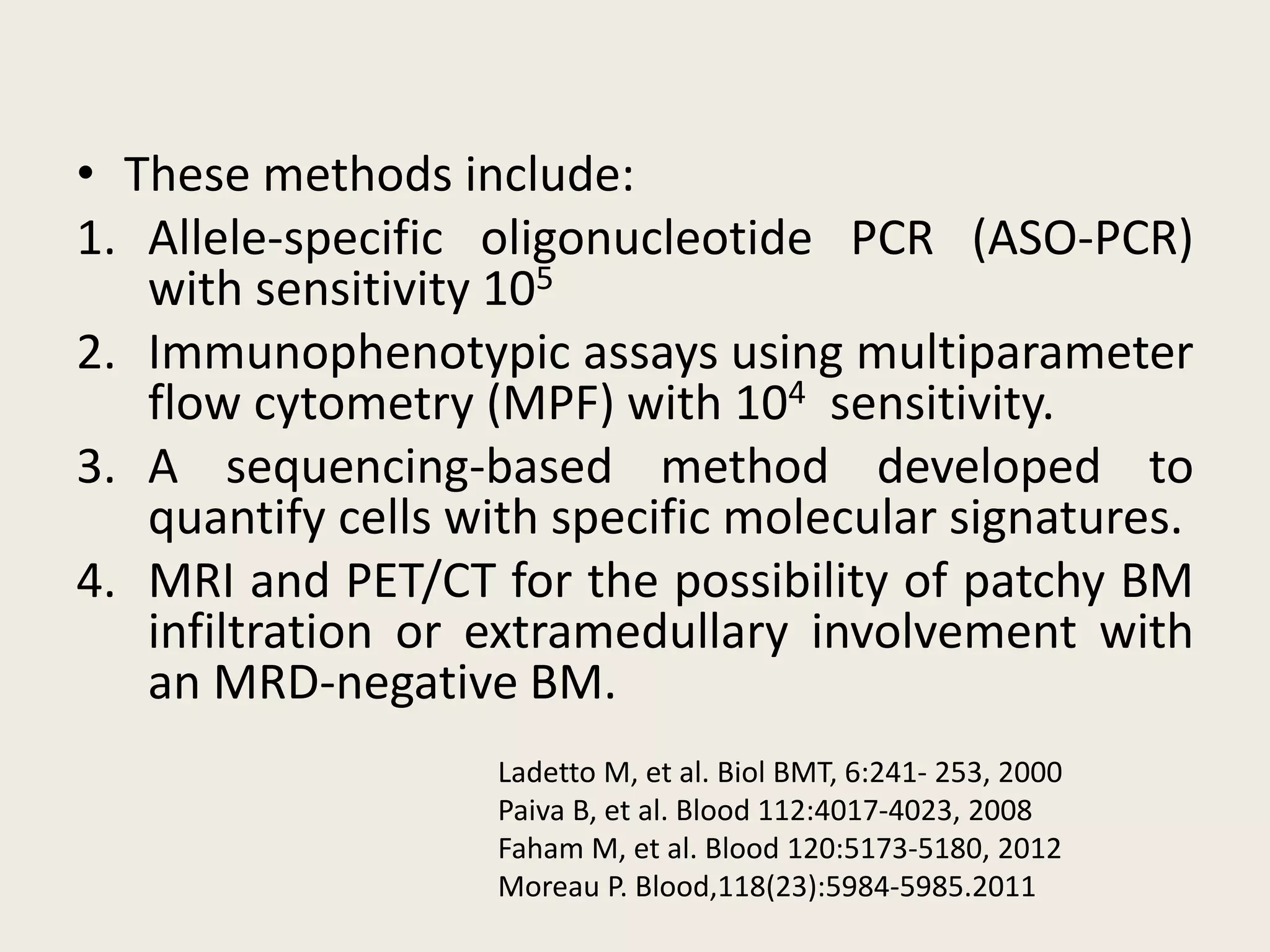
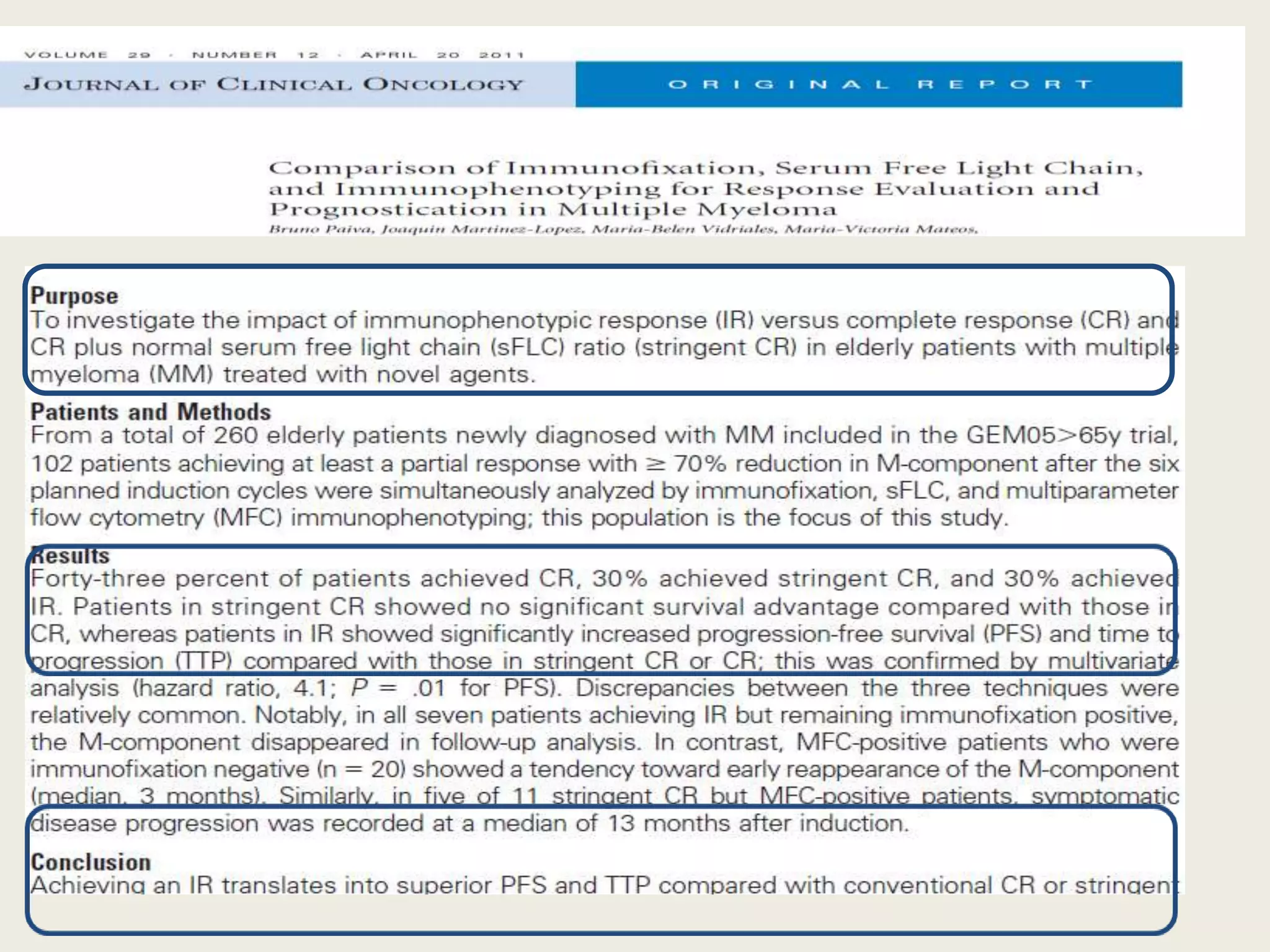
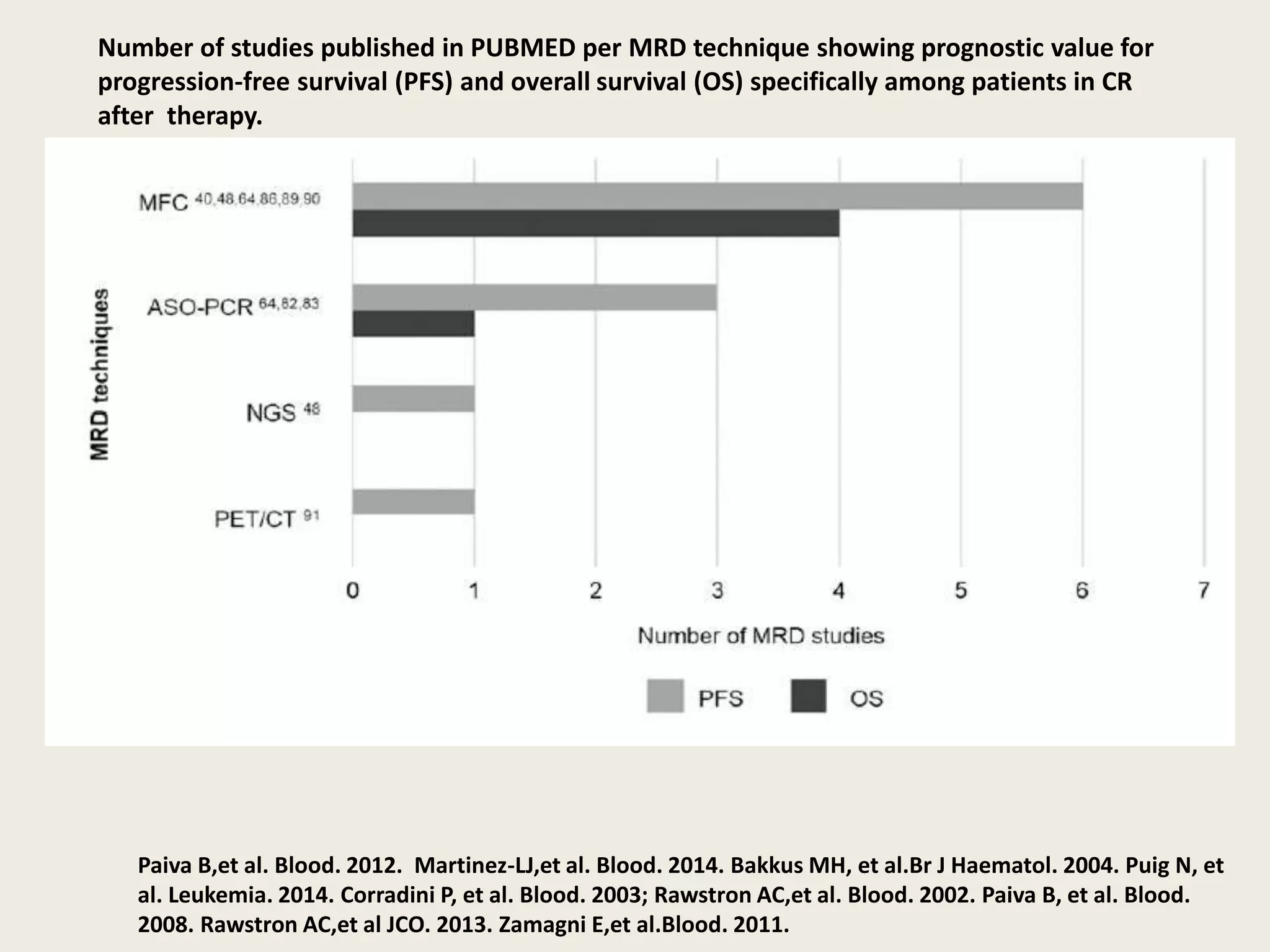
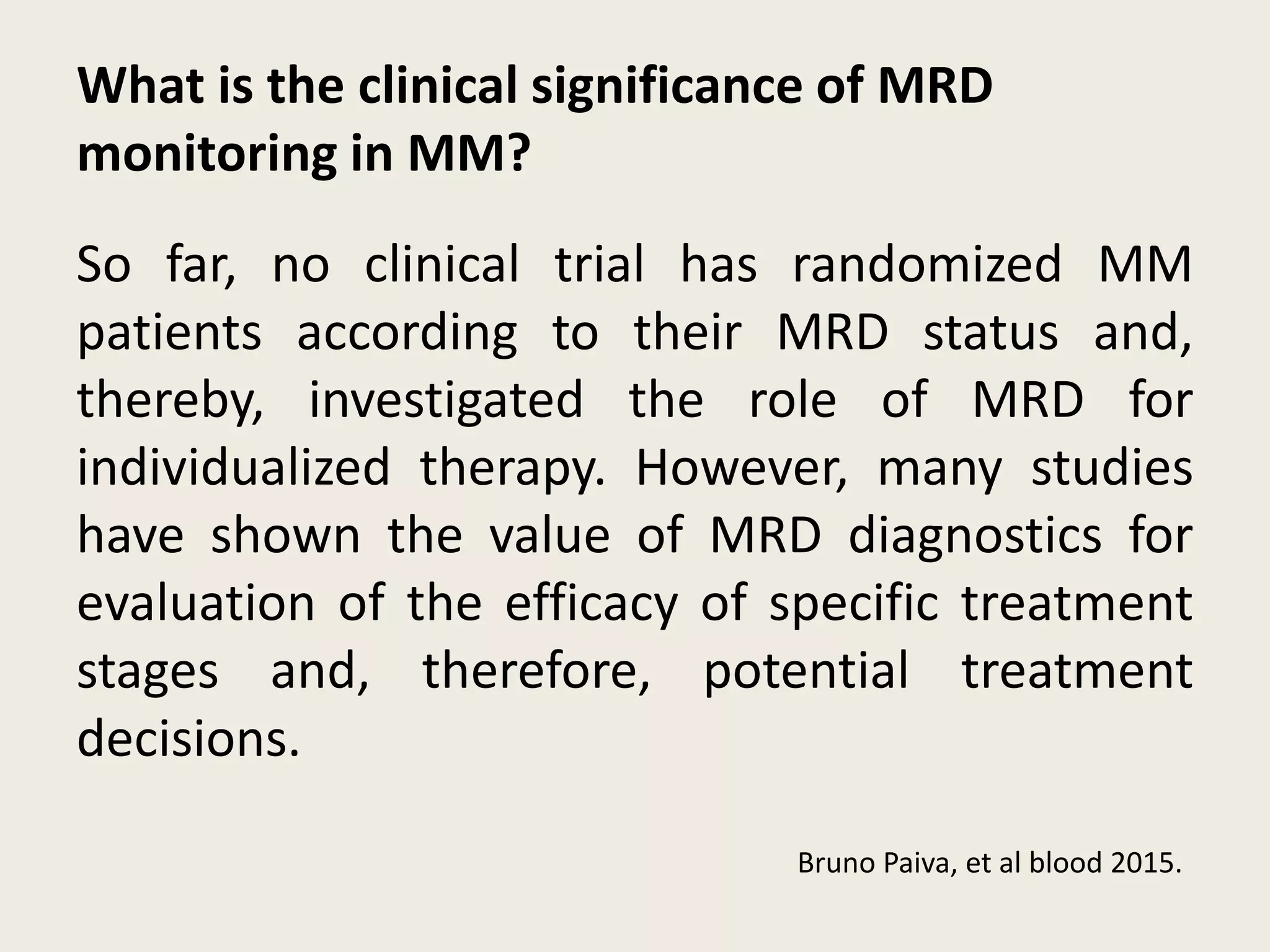
This document presents the case of a 59-year-old woman who presented with low back pain. Imaging showed osteolytic bone lesions and a bone marrow biopsy revealed 20% plasma cell infiltration, confirming a diagnosis of multiple myeloma. She was started on CyBorD chemotherapy and achieved a complete response. She then underwent autologous stem cell transplantation with conditioning using melphalan, followed by thalidomide maintenance therapy. Currently, she is doing well with no evidence of multiple myeloma on follow up testing and is tolerating the thalidomide maintenance treatment.