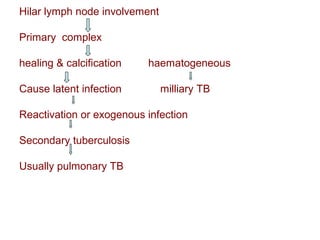
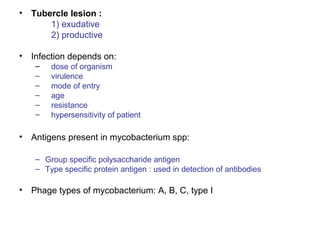
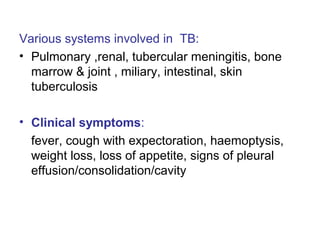
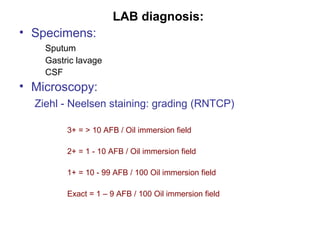
Mycobacteria are a group of bacteria that can cause diseases like tuberculosis and leprosy. There are typical mycobacteria that are obligate pathogens like M. tuberculosis, as well as atypical mycobacteria that are opportunistic pathogens. Laboratory tests are used to identify mycobacteria species and diagnose diseases, while treatments involve prolonged use of multiple antibiotics. Dental professionals should be aware of atypical mycobacteria infections as they can occasionally present as oral lesions.