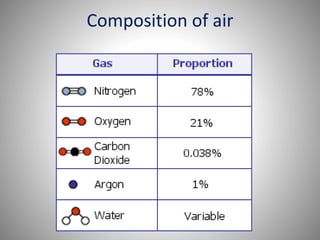
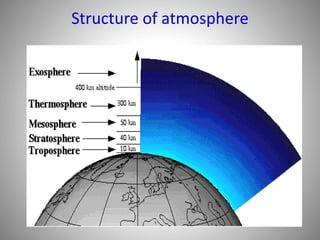
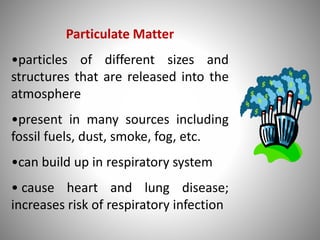
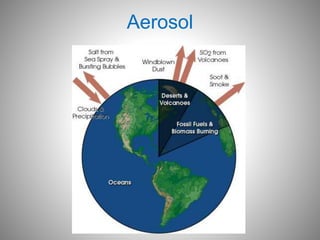
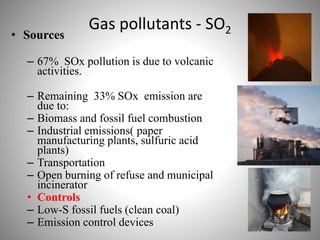
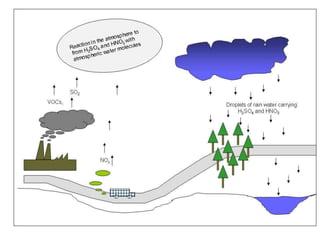
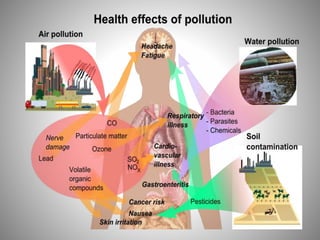
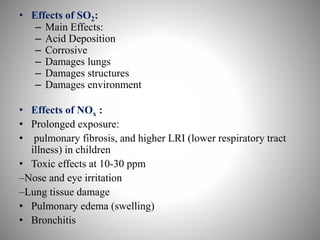
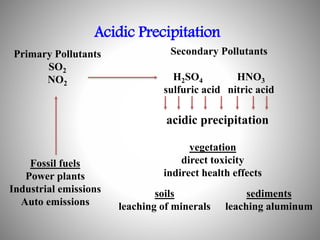
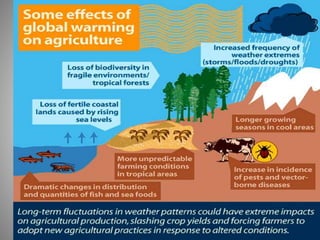
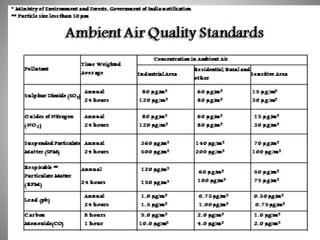
The document provides a comprehensive overview of air pollution, covering its definition, composition, classifications, sources (both natural and anthropogenic), and health and environmental effects. Key air pollutants discussed include sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter, highlighting their sources, impacts on human health and vegetation, and contributions to climate change. It also addresses the adverse effects of air pollution on materials and property, and emphasizes the importance of understanding and controlling air quality.