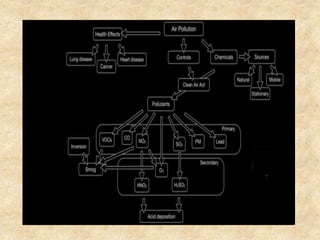
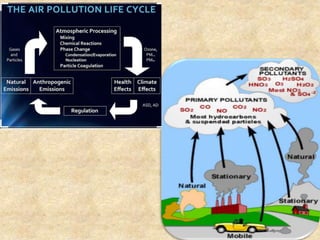
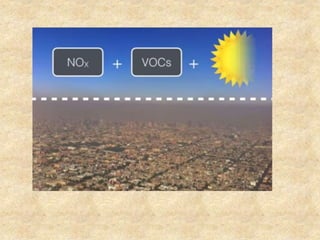
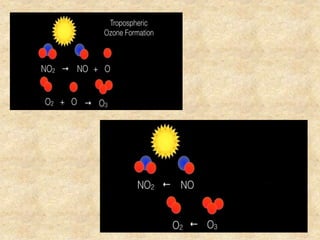
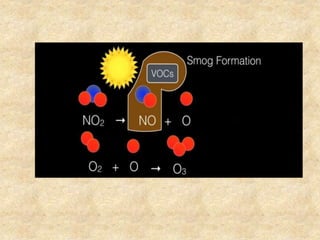
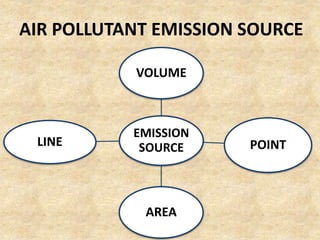
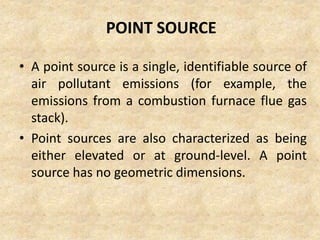
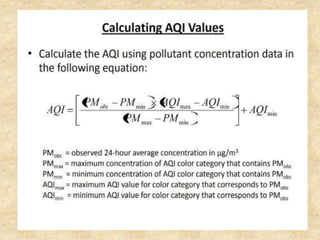
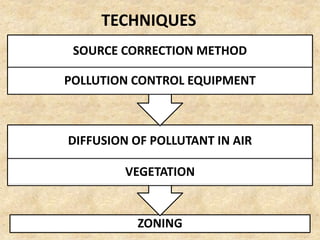
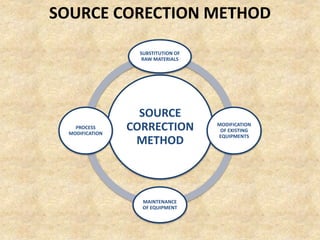
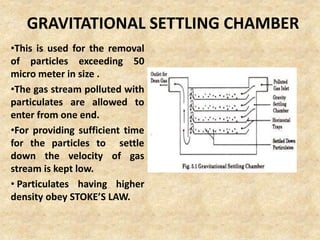
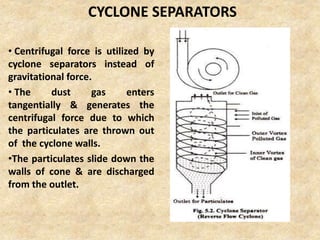
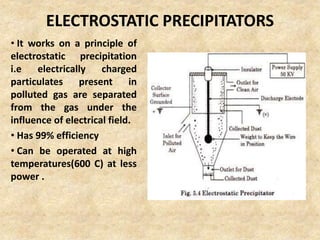
This document discusses various air pollutants and their sources. It identifies the most common primary pollutants like particulate matter, nitrogen dioxide, sulfur dioxide, carbon monoxide and carbon dioxide. Secondary pollutants formed from these primary ones include smog, ground level ozone and peroxyacetyl nitrate. Major sources of air pollution are described as point sources like smokestacks, line sources like traffic, area sources like landfills and volume sources like industrial facilities. Specific pollutants like sulfur and nitrogen oxides from fossil fuel combustion, carbon monoxide from vehicles and ozone formation from vehicle emissions are explained. Health effects from particulate matter and gases like irritation and worsening of lung/heart diseases are summarized.