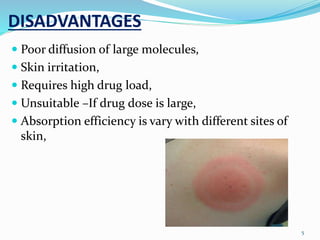
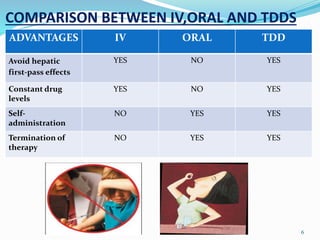
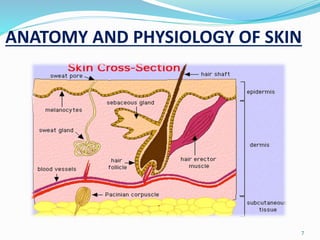
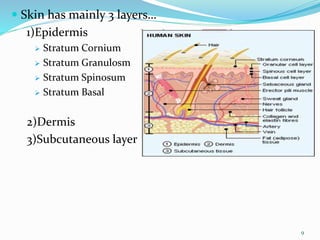
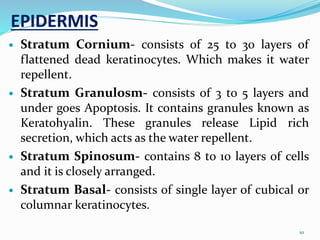
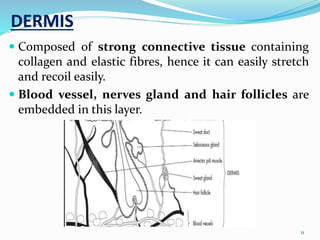
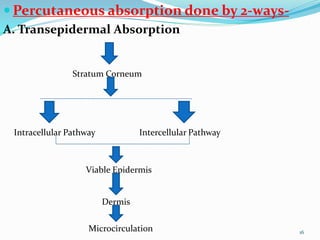
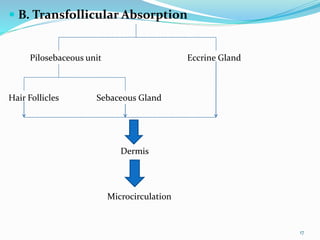
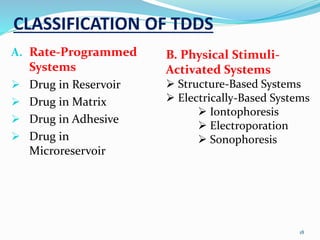
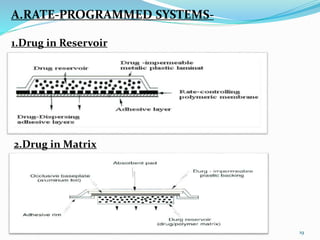
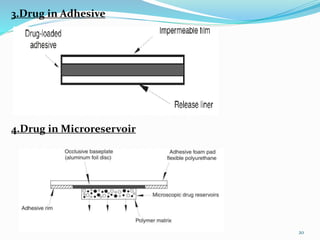
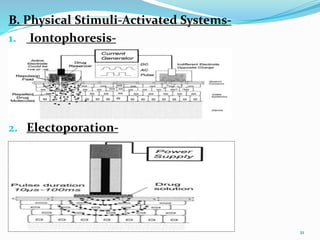
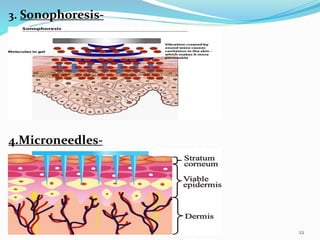
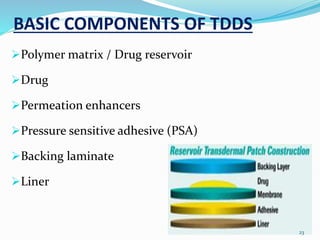
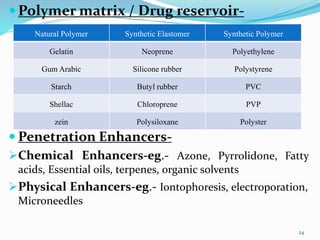
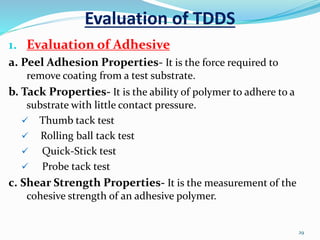
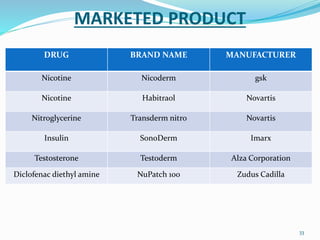
This document provides an overview of transdermal drug delivery systems (TDDS). It discusses how TDDS work by delivering drugs through the skin for systemic effects at predetermined rates. The key advantages of TDDS include avoiding first-pass metabolism, providing long-lasting drug levels comparable to IV infusion, and allowing easy termination of drug delivery. The document outlines the anatomy and physiology of the skin, drug permeation through skin, and factors affecting permeation. It also describes various TDDS classifications, components, evaluation methods, applications, and some marketed TDDS products.