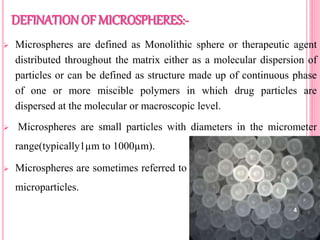
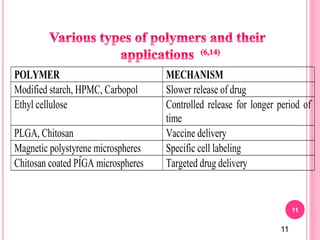
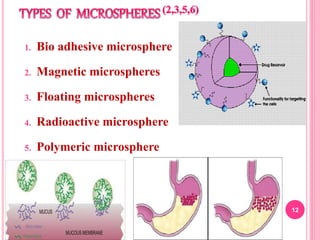
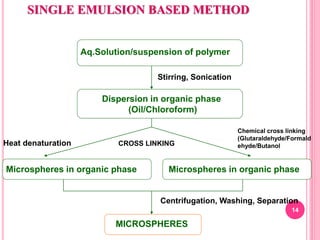
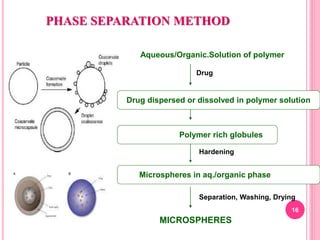
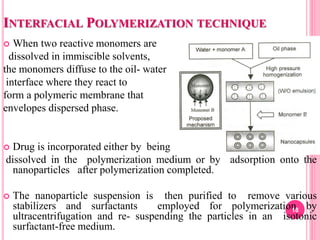
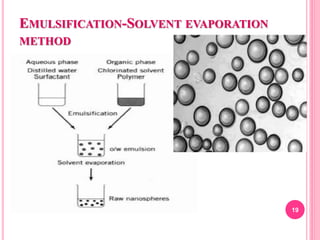
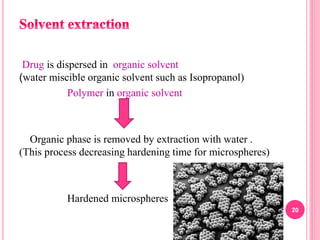
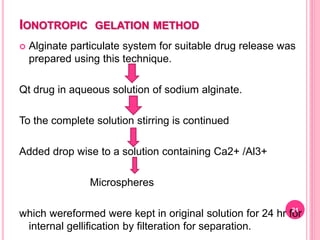
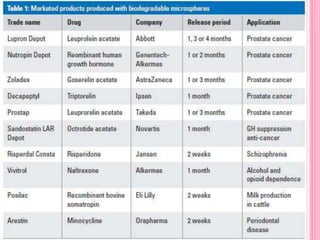
The document discusses microspheres as a drug delivery system. It defines microspheres as small spherical particles ranging from 1μm to 1000μm that can be used to deliver drugs in a sustained, controlled release fashion. Various methods for producing microspheres are described, including single emulsion, double emulsion, phase separation, spray drying, and ionotropic gelation. The properties, mechanisms, types, and applications of microspheres are summarized. Evaluation methods for microspheres such as particle size, drug loading, and in vitro release are also outlined.