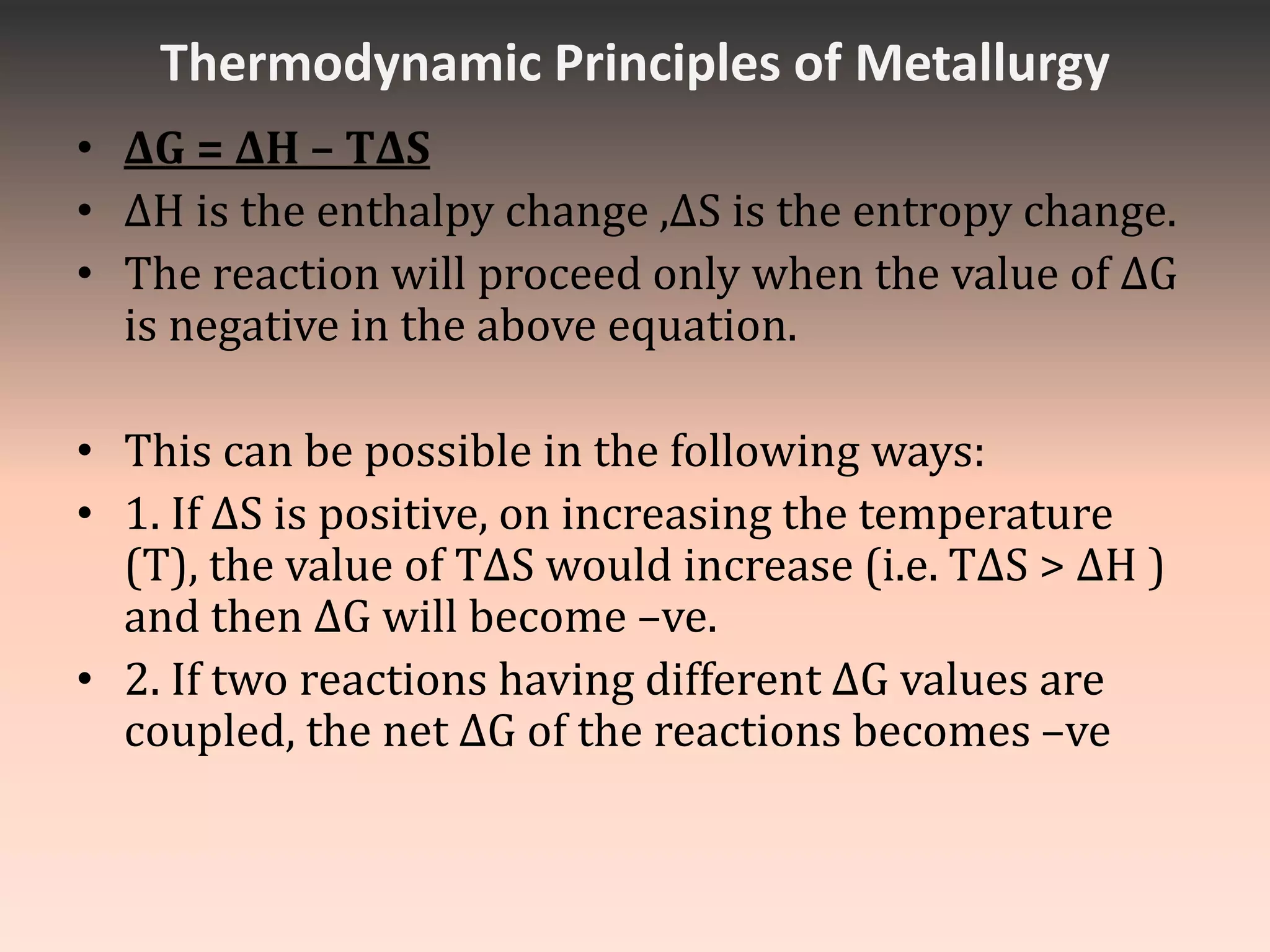
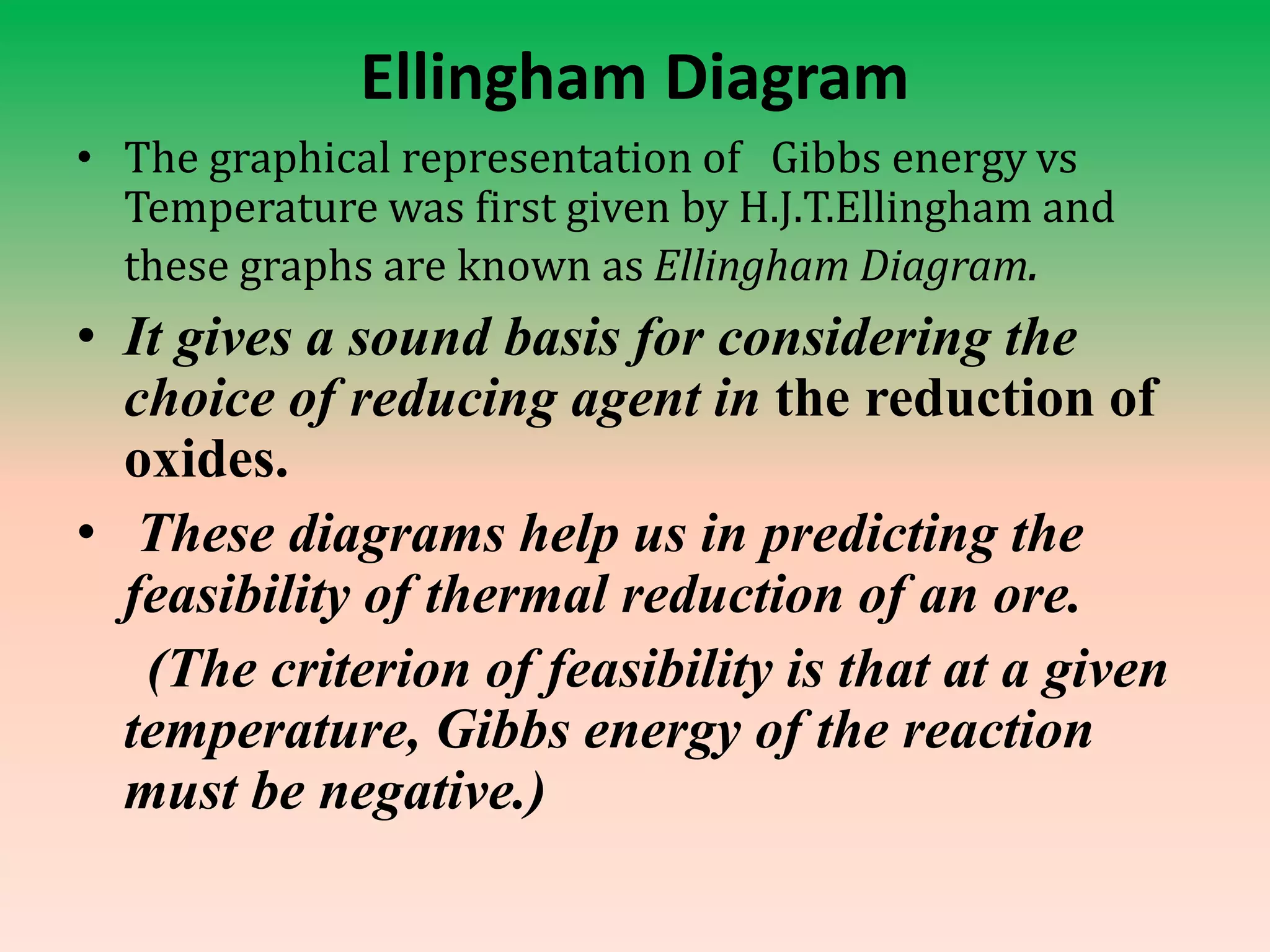
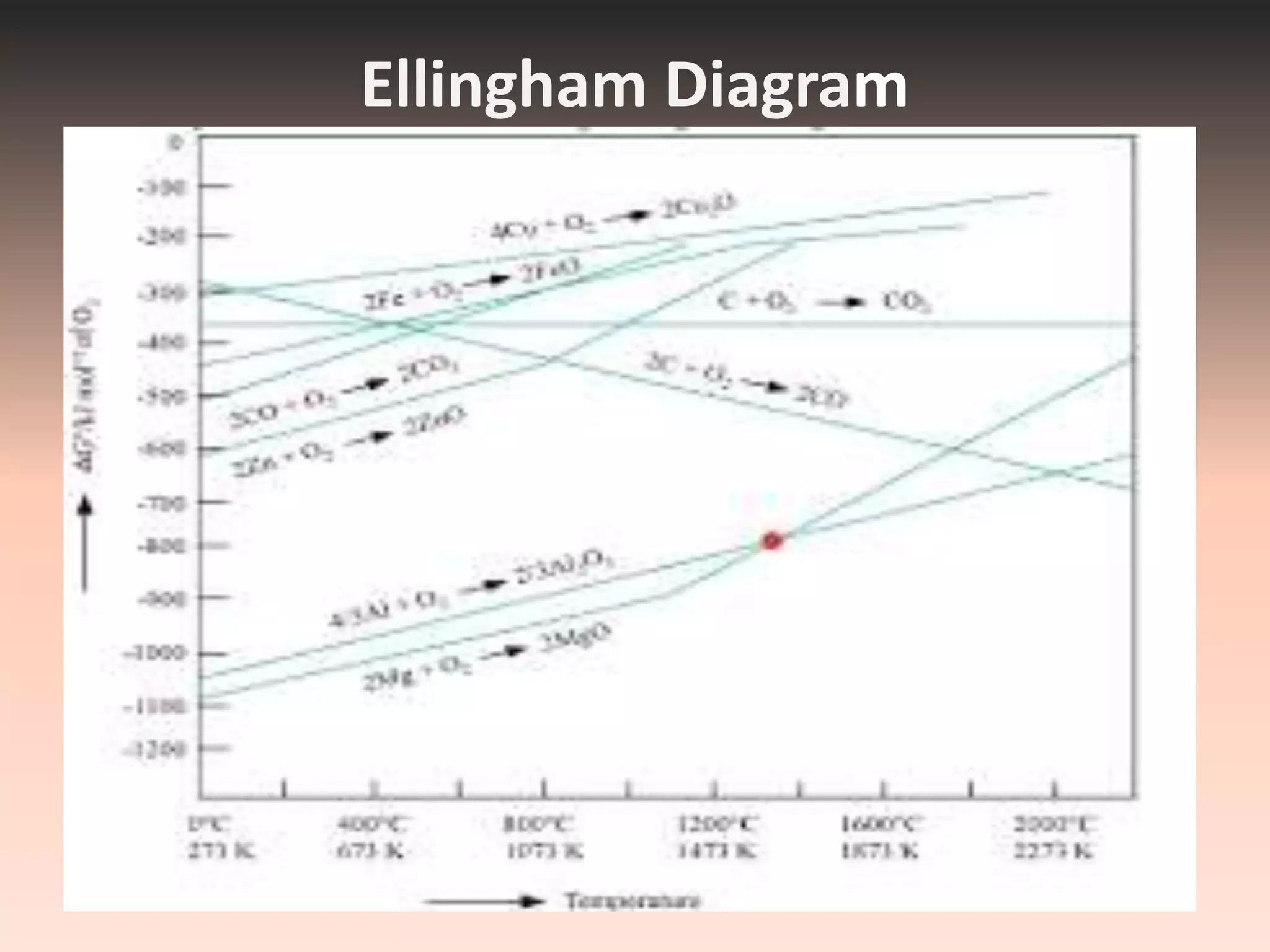
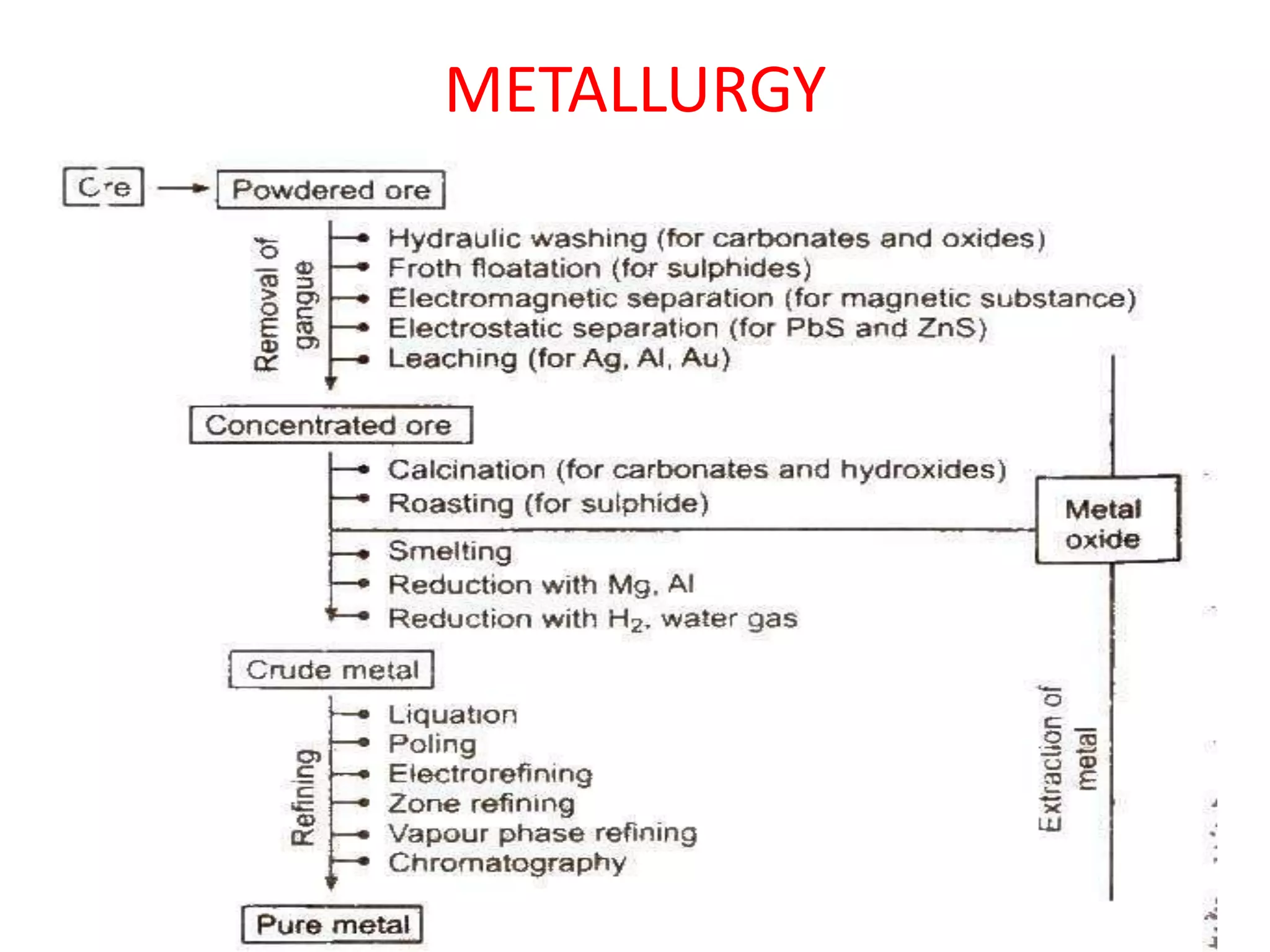
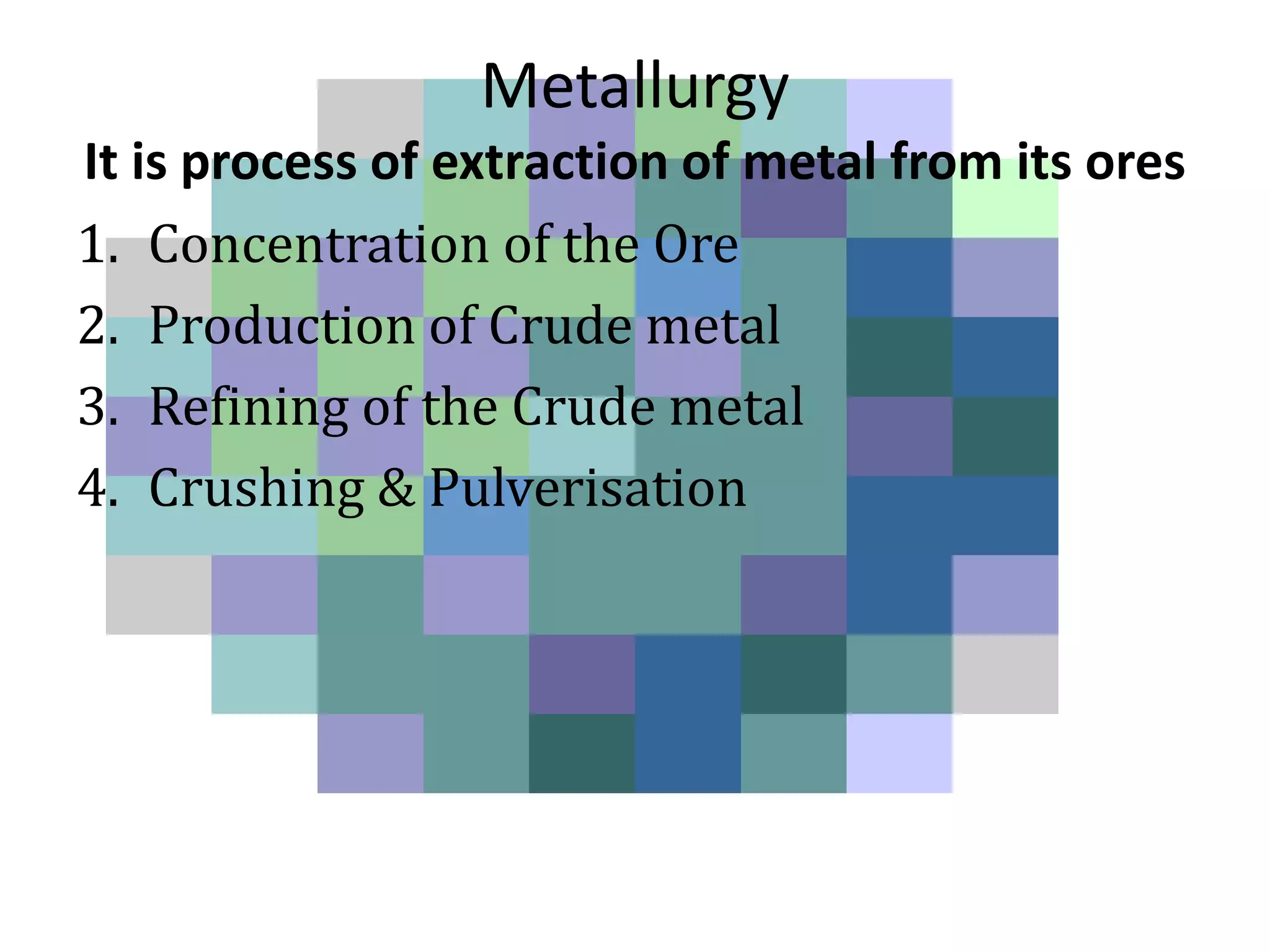
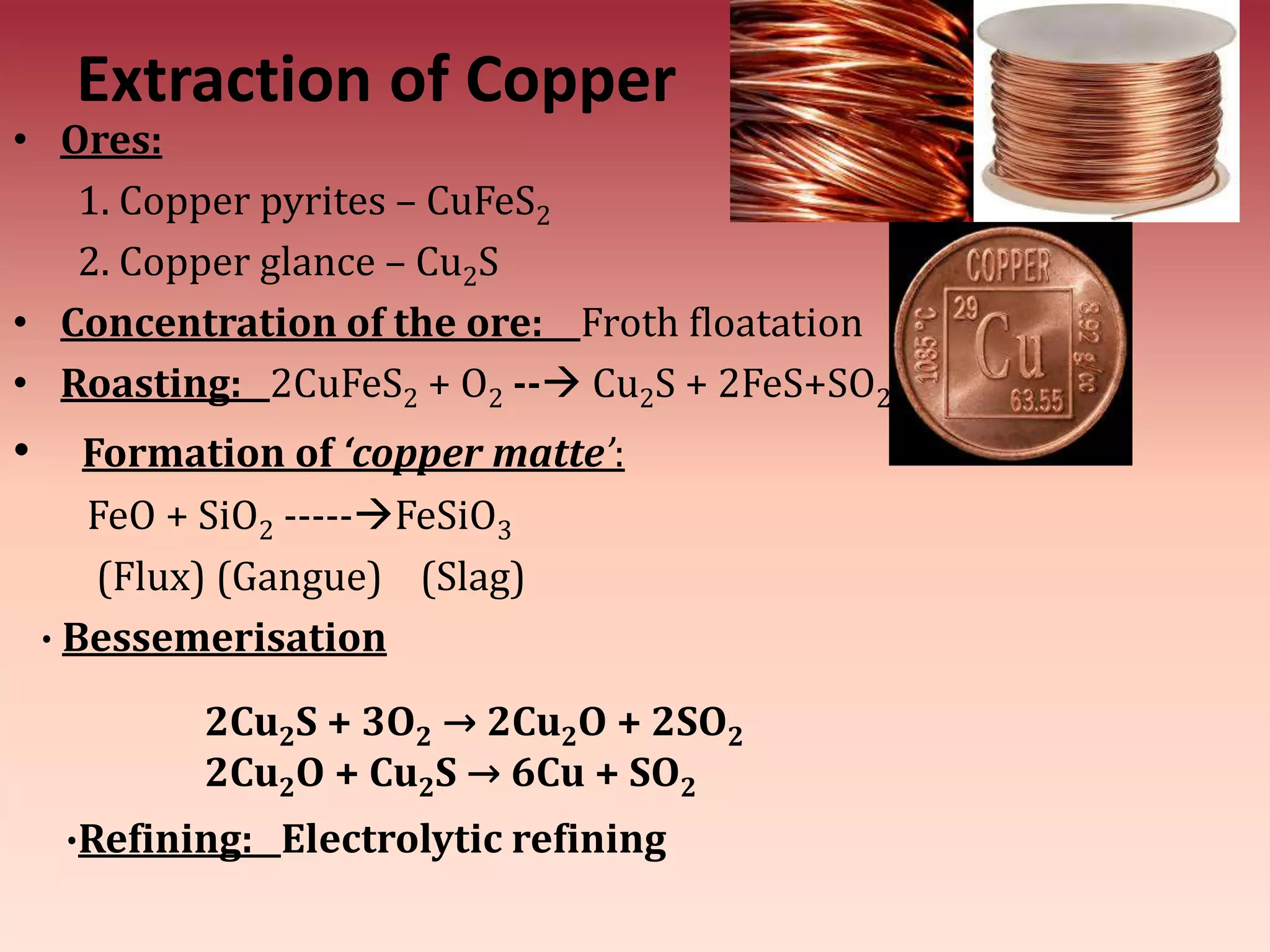
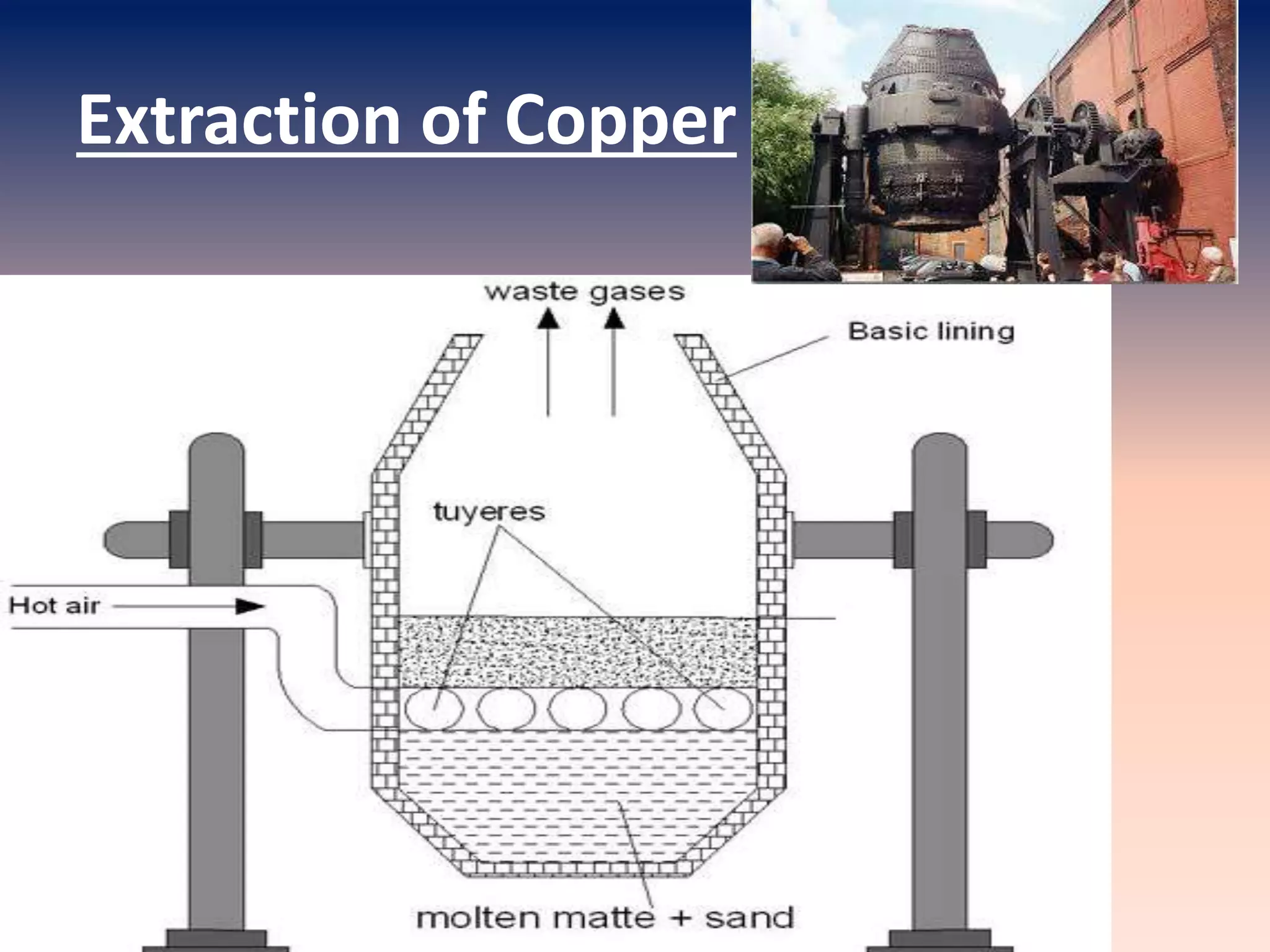
This document discusses metallurgy and metal extraction processes. It covers key topics such as mineral ores, concentration methods like froth flotation and magnetic separation, production of crude metal through processes like smelting and calcination, and refining of crude metal using techniques including liquation, distillation, and electrorefining. Specific extraction processes are described for metals like aluminum, copper, iron, and zinc. Thermodynamic principles and the use of Ellingham diagrams to determine the feasibility of thermal reduction reactions are also summarized.





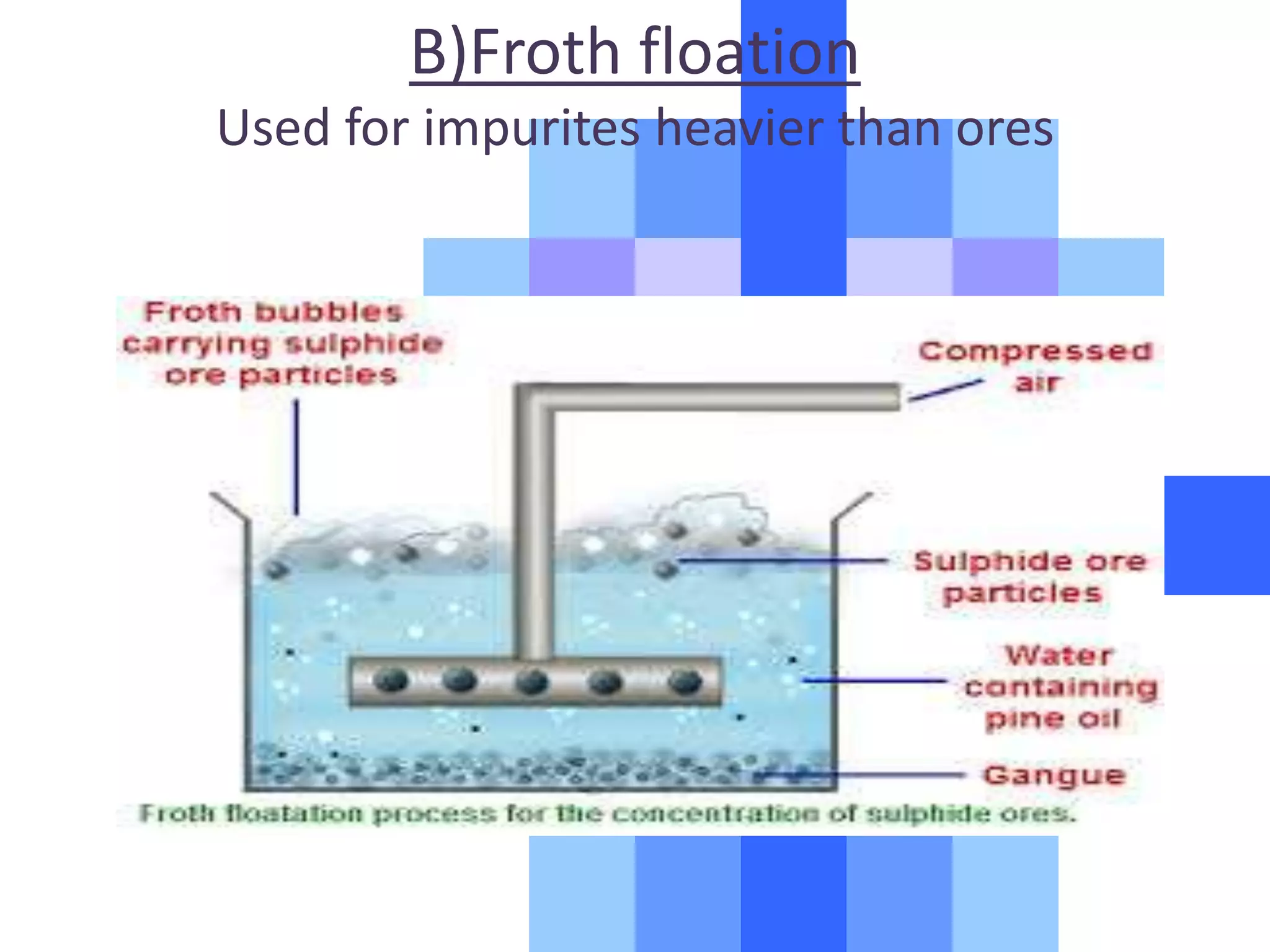
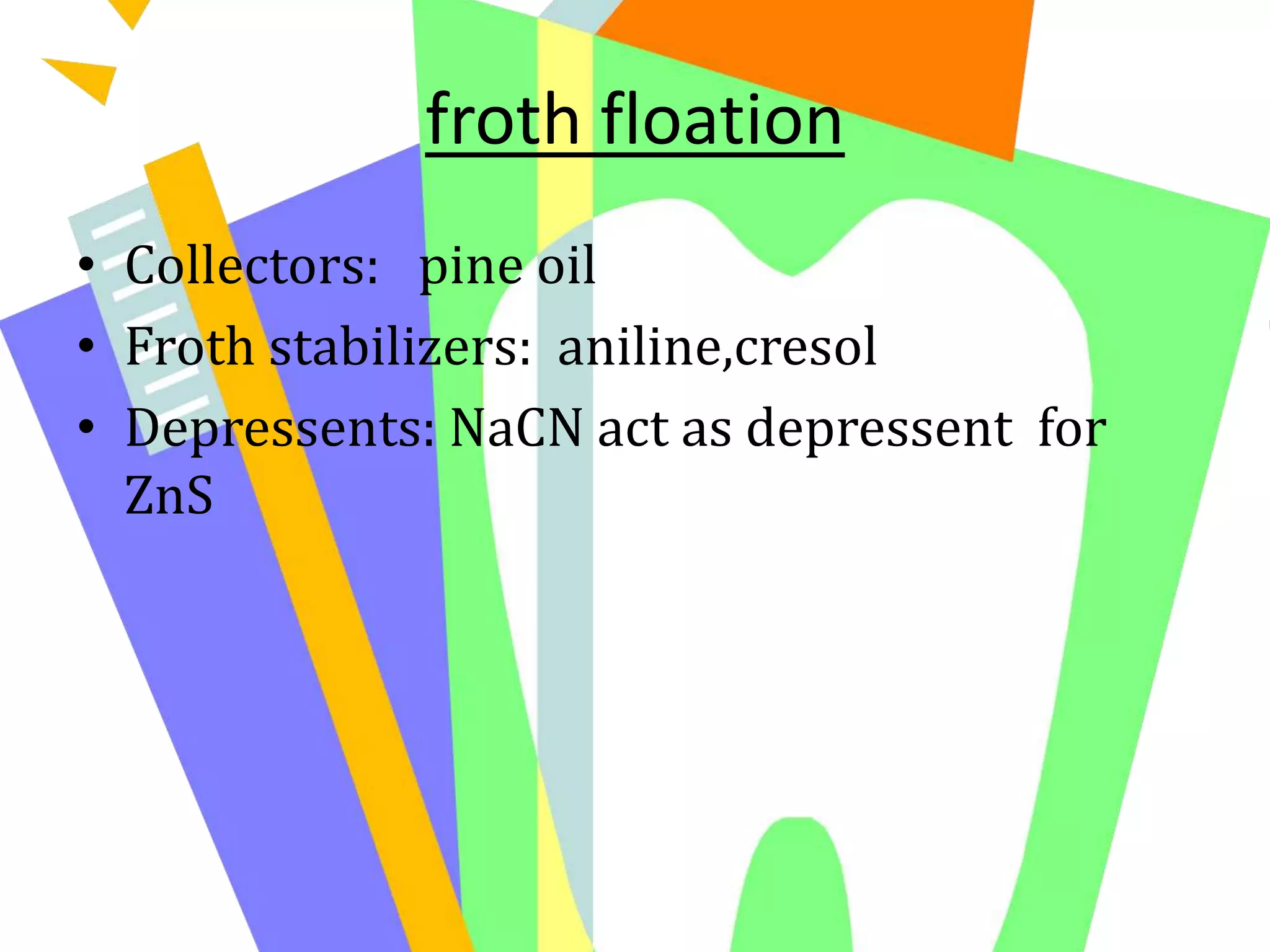
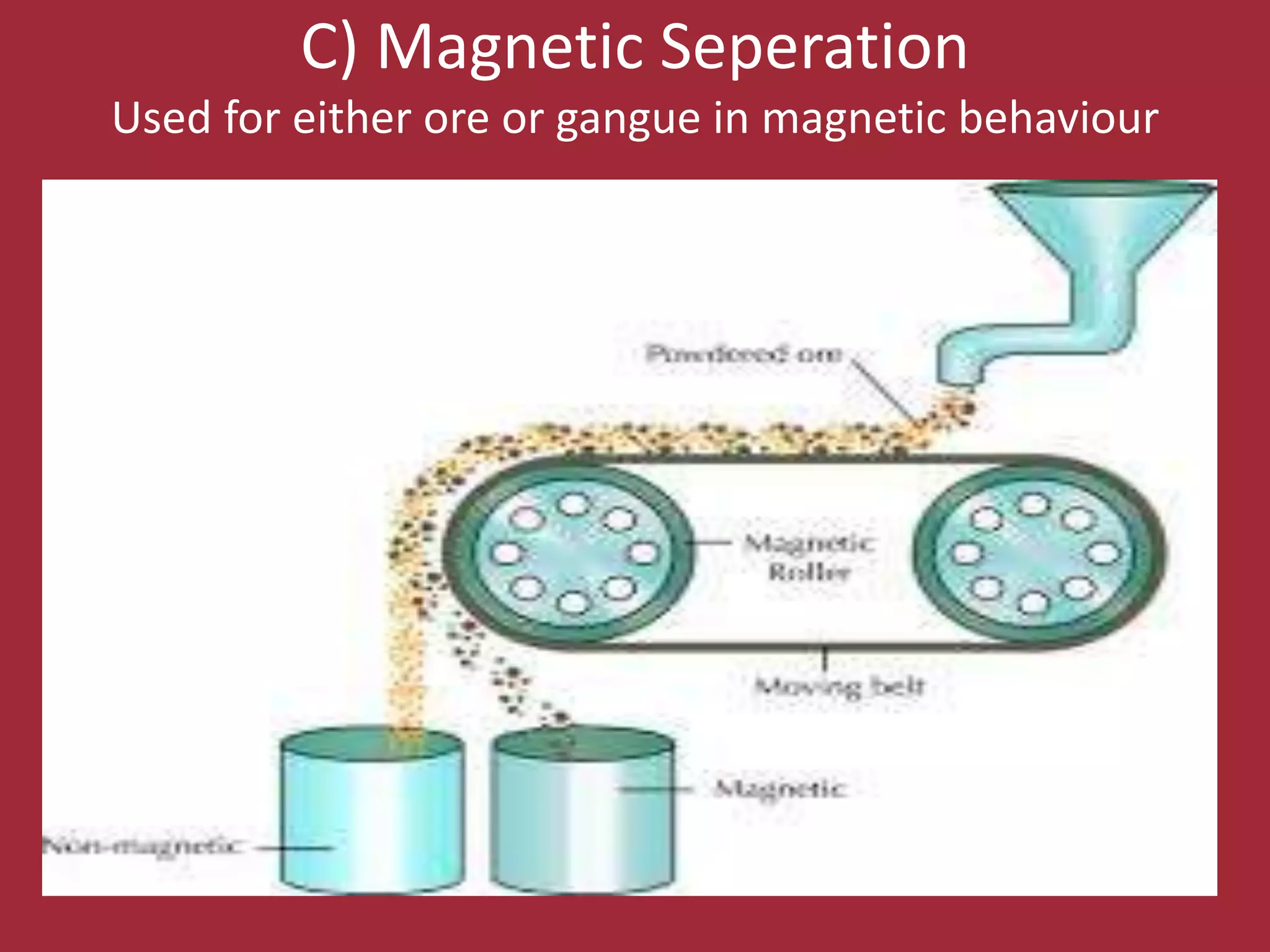
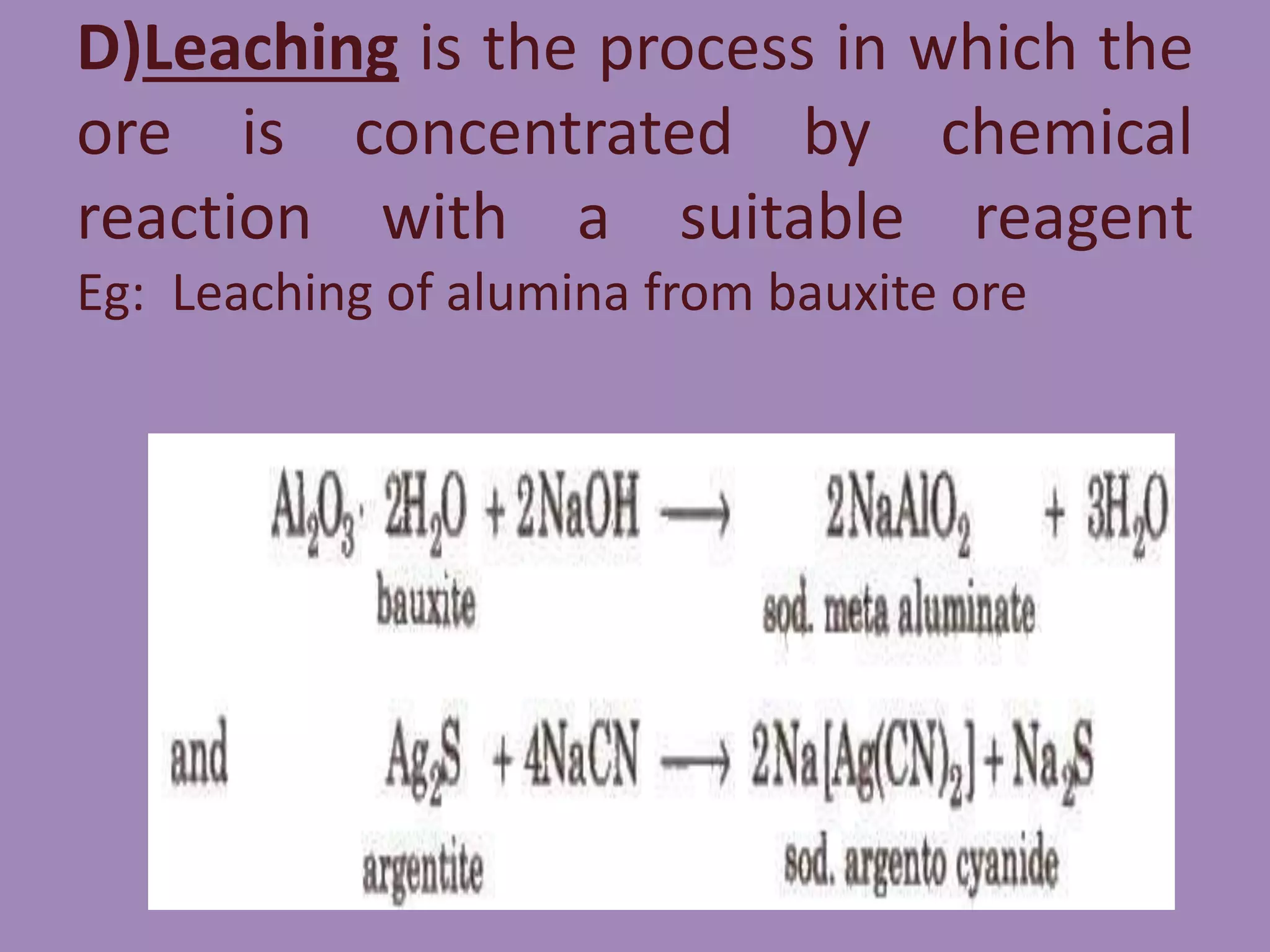
![. Reduction of the oxides to metal
• (i) Smelting (reduction with carbon)
• Flux: During smelting a substance. called flux is
added which removes the non-fusible impurities
as fusible slag. [Flux + Gangue = Slag]
• Acidic flux For basic impurities, acidic flux is
added.
• e.g., CaO + SiO2 → CaSiO3](https://image.slidesharecdn.com/metallurgy2-190128085700/75/Metallurgy-based-on-chemistry-the-reactions-and-processes-14-2048.jpg)
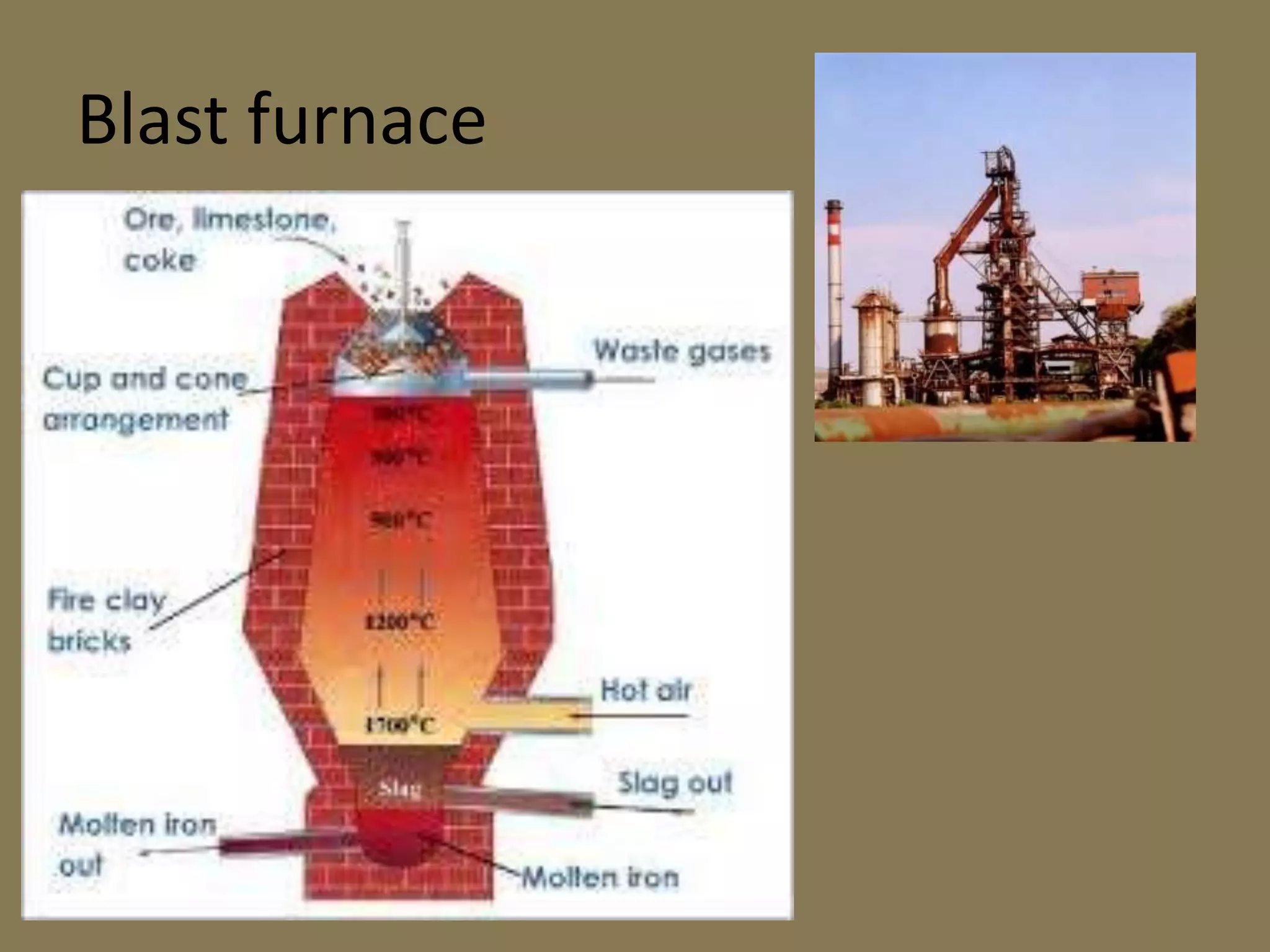
![Extraction of Iron
ores: Haematite (Fe2O3) and Magnetite (Fe3O4).
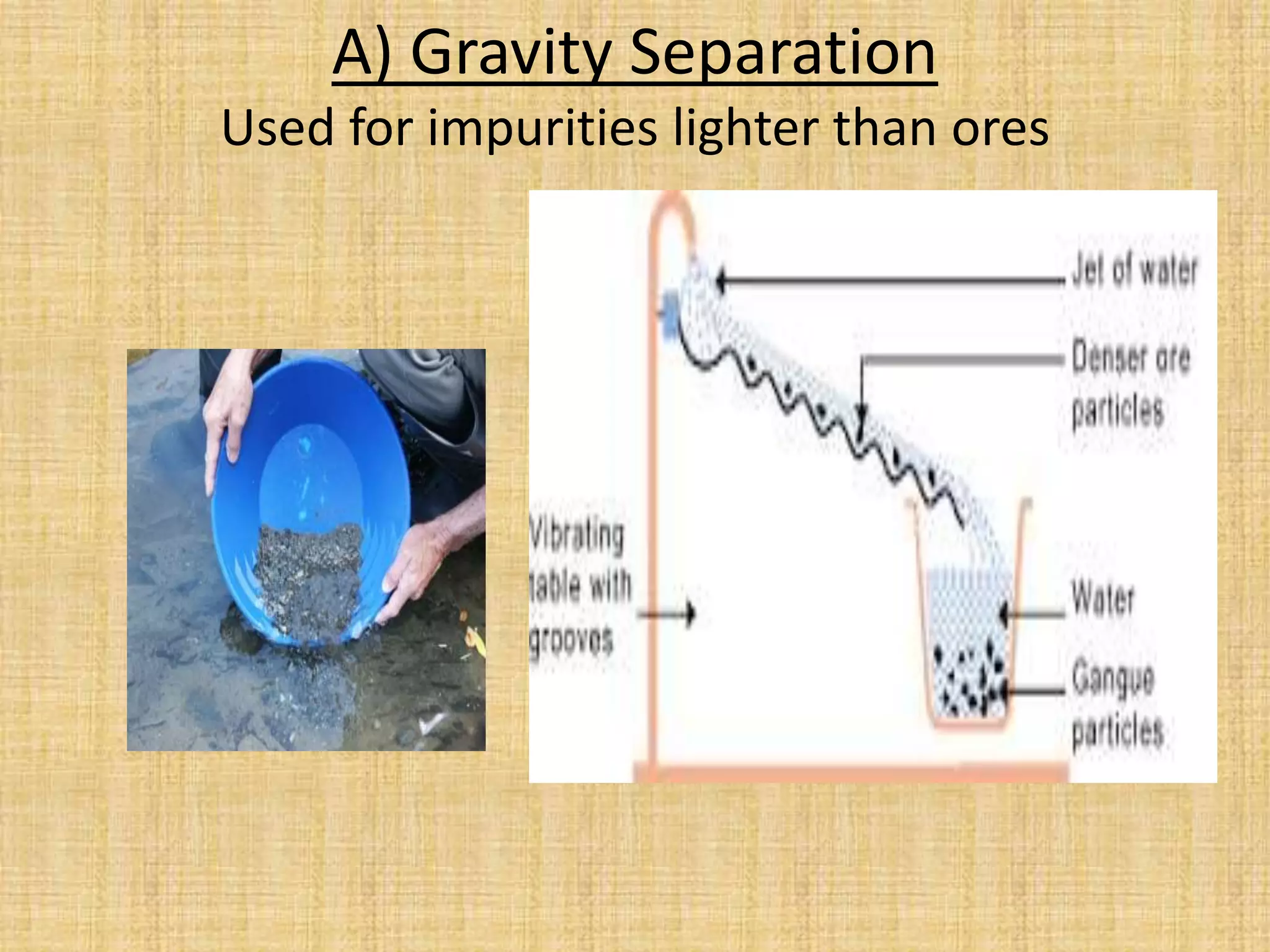
Concentration of the ore: Gravity Separation
Calcination :
Reduction of the ore:
2 C(s) + O2(g) → 2 CO2(g+ Heat [ Combustion Zone] (1775K)
C(s) + CO2(g) → 2 CO(g [ Fusion Zone] (1575K)
CaCO3 CaO + CO2 [Slag Formation Zone] (1275K)
CaO + SiO2 CaSiO3
3Fe2O3 + CO → 2 Fe3O4 + CO2 [Reduction Zone] (875K)
Fe3O4 + 4 CO → 3Fe + 4 CO2o
FeO(s) + CO(g) → Fe(s) + CO2(g](https://image.slidesharecdn.com/metallurgy2-190128085700/75/Metallurgy-based-on-chemistry-the-reactions-and-processes-24-2048.jpg)