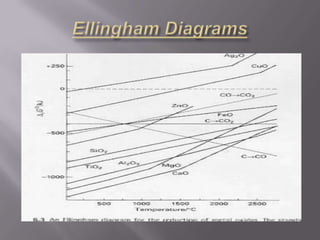
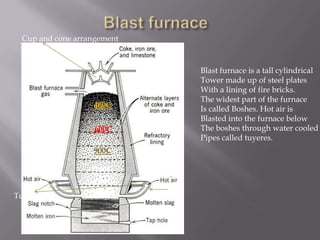
Metallurgy is the process of extracting pure metals from their ores. Ores contain unwanted impurities and the required metal. The process involves both physical and chemical steps to separate the metal from impurities. One such process is the Parke's process for extracting silver, which involves heating an alloy of lead and silver to melt it, mixing it with molten zinc where the silver dissolves and separates from the lead due to different solubilities. Ellingham diagrams graphically show the thermodynamic stability of metal oxides and their variation with temperature, indicating the spontaneity of oxidation reactions. Iron is extracted from its ore, haematite, using a blast furnace where coke and limestone are added and hot