Embed presentation
Download to read offline


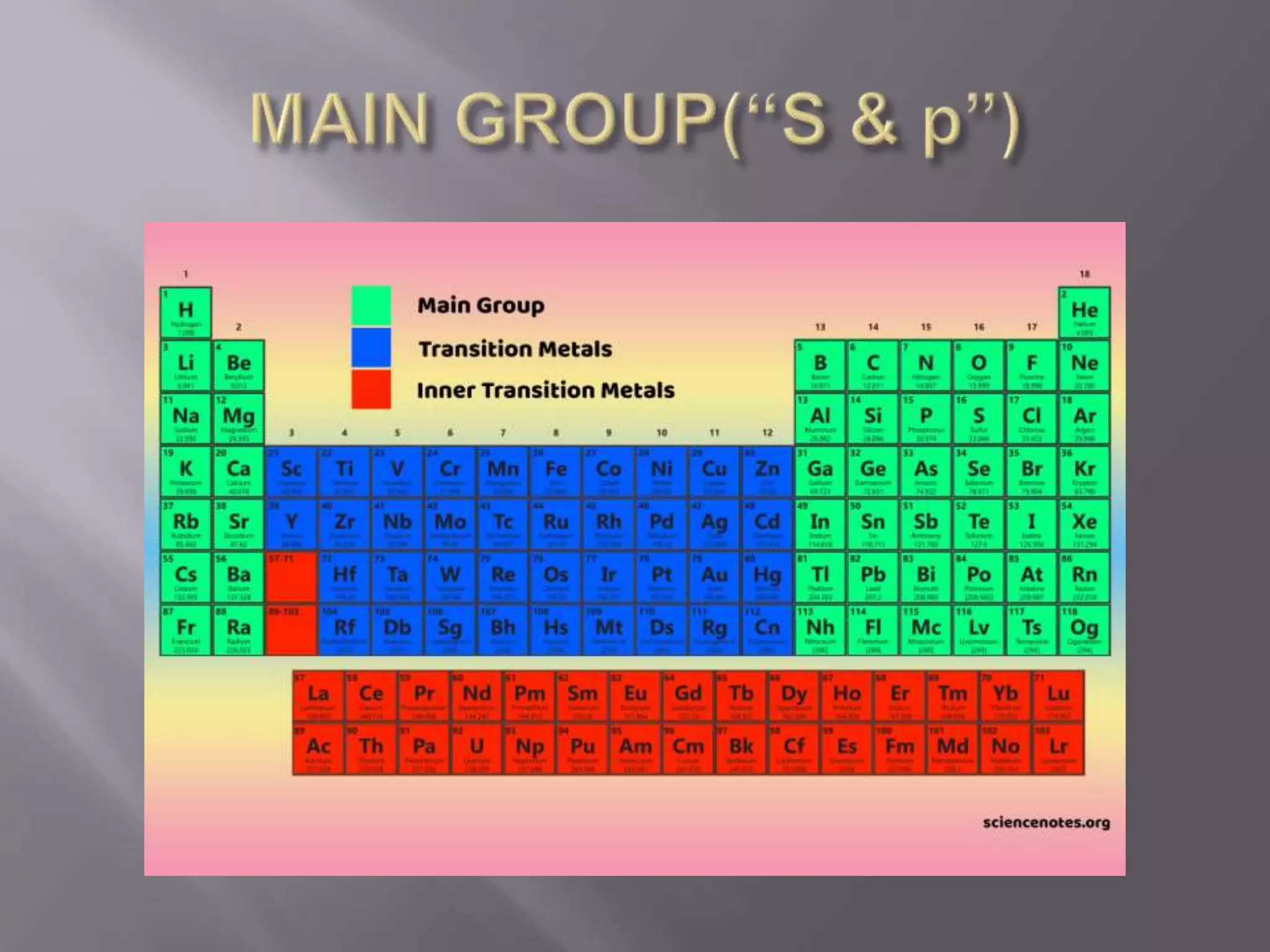
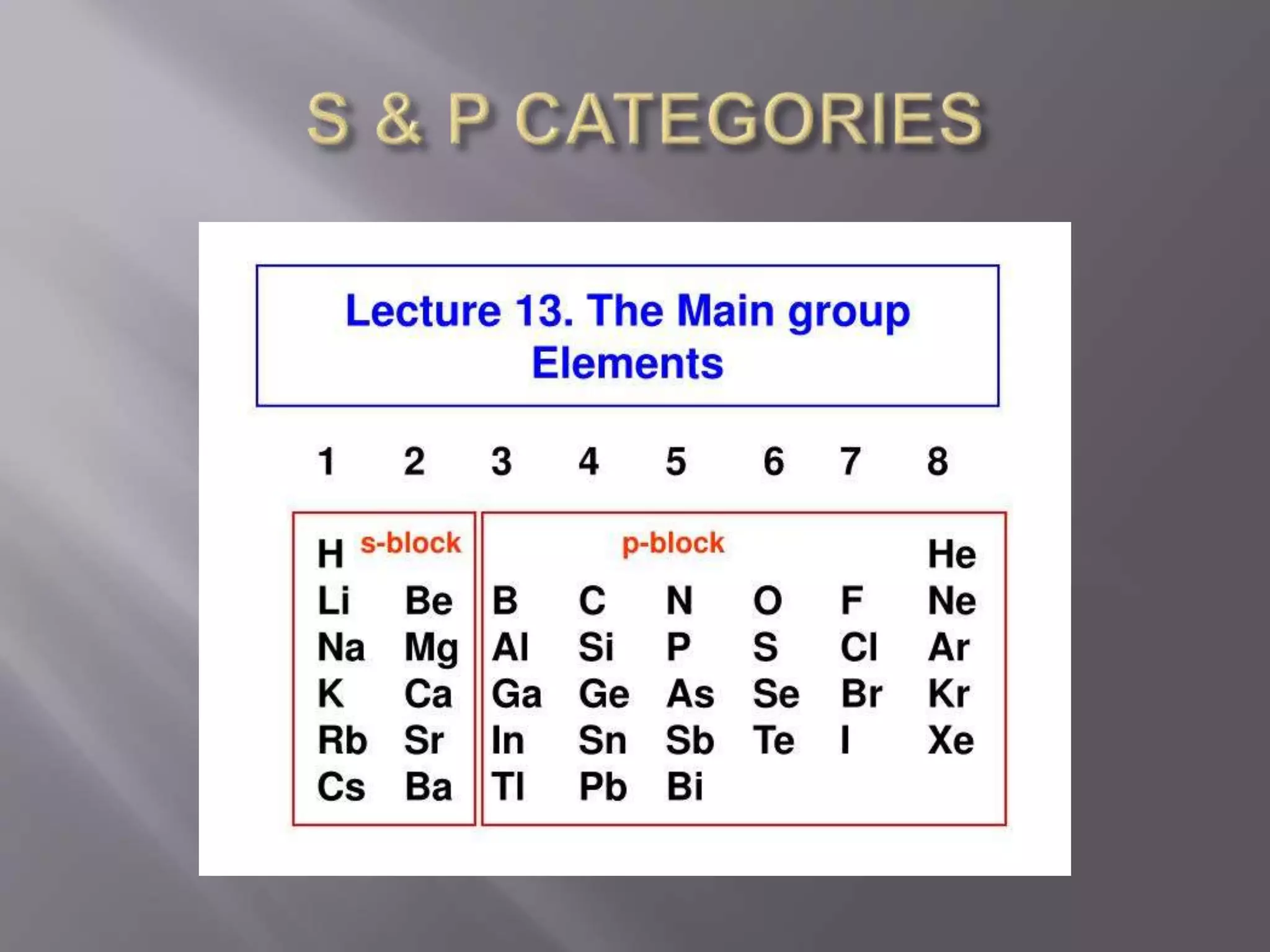
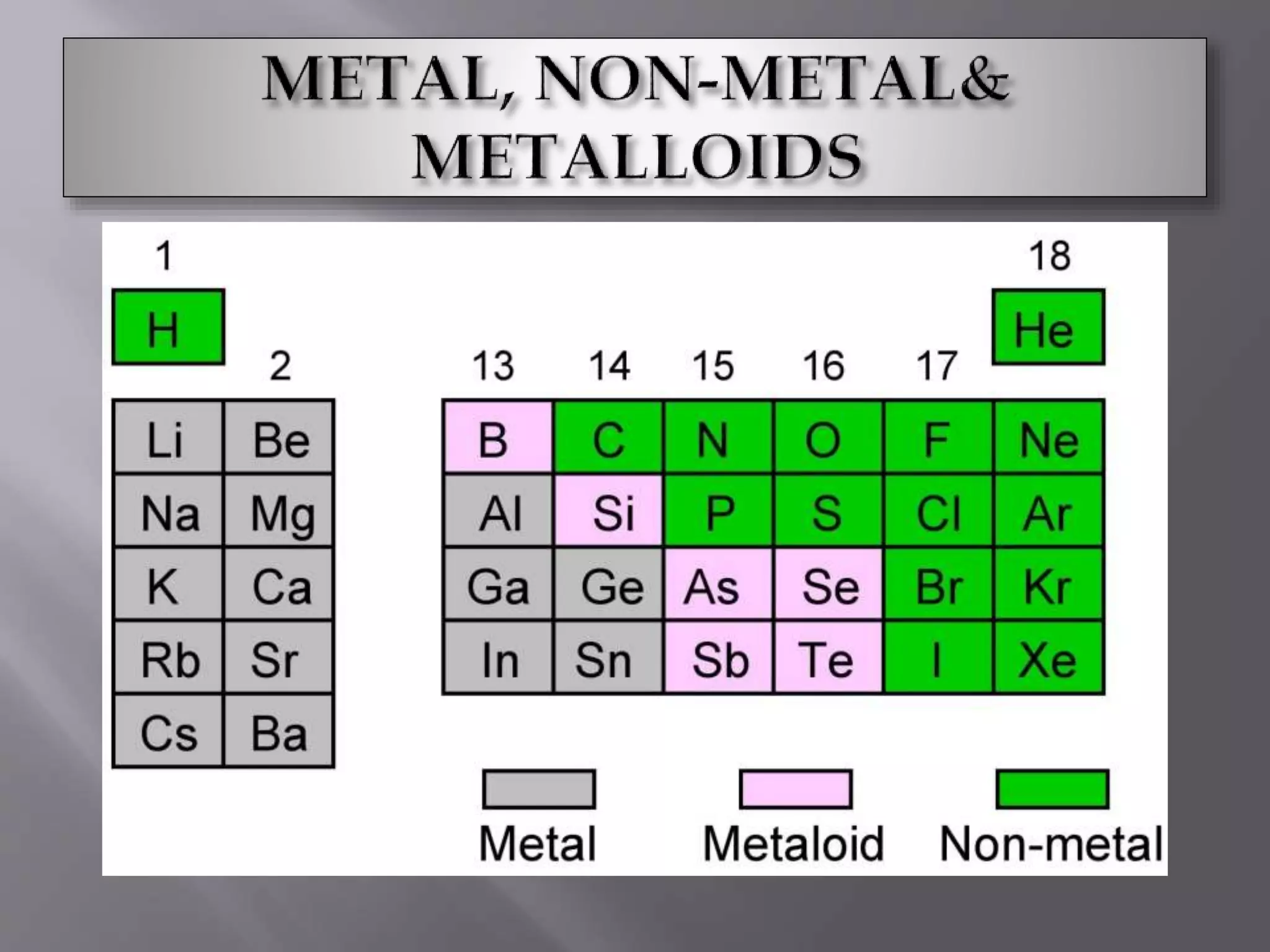


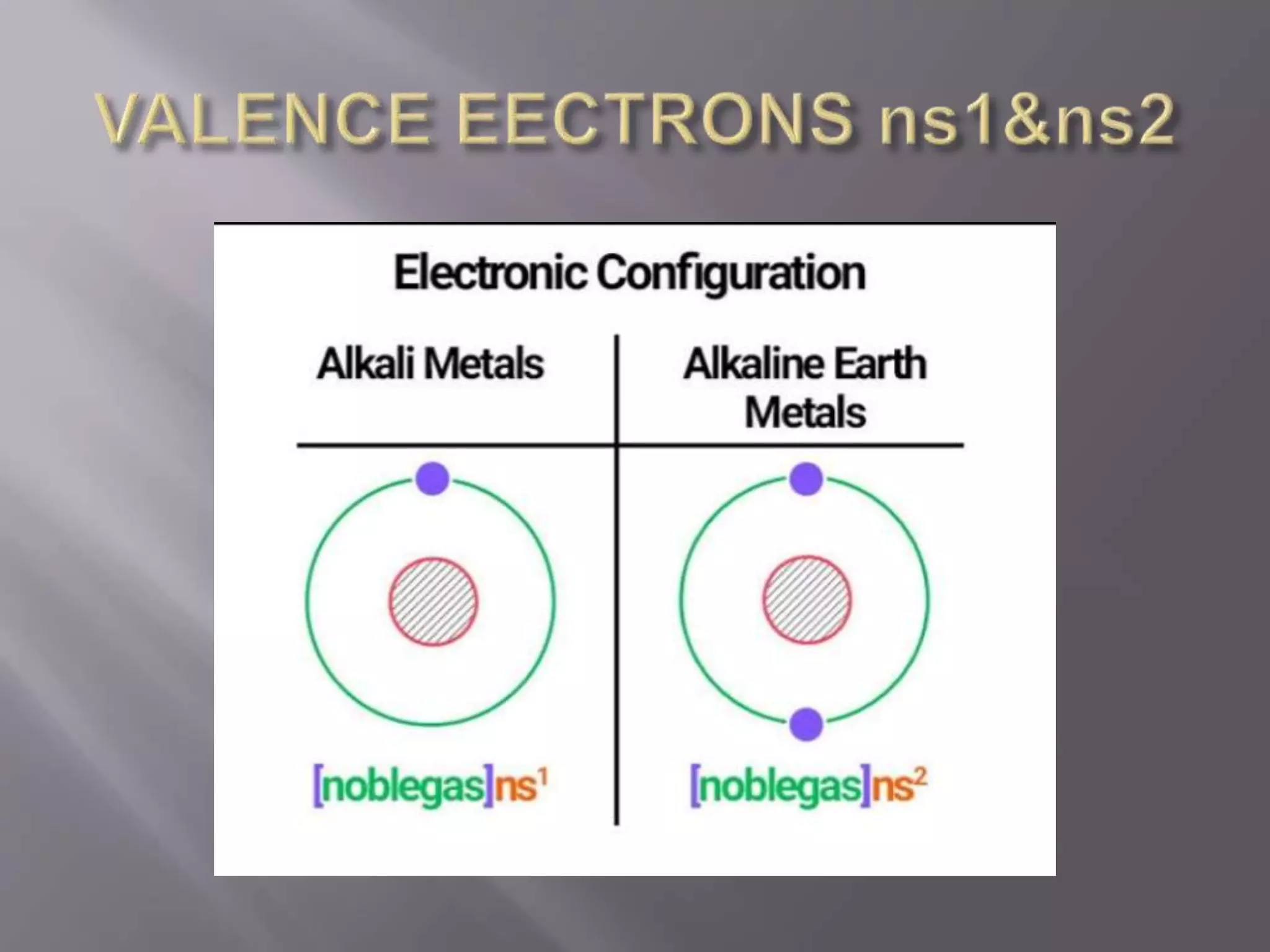
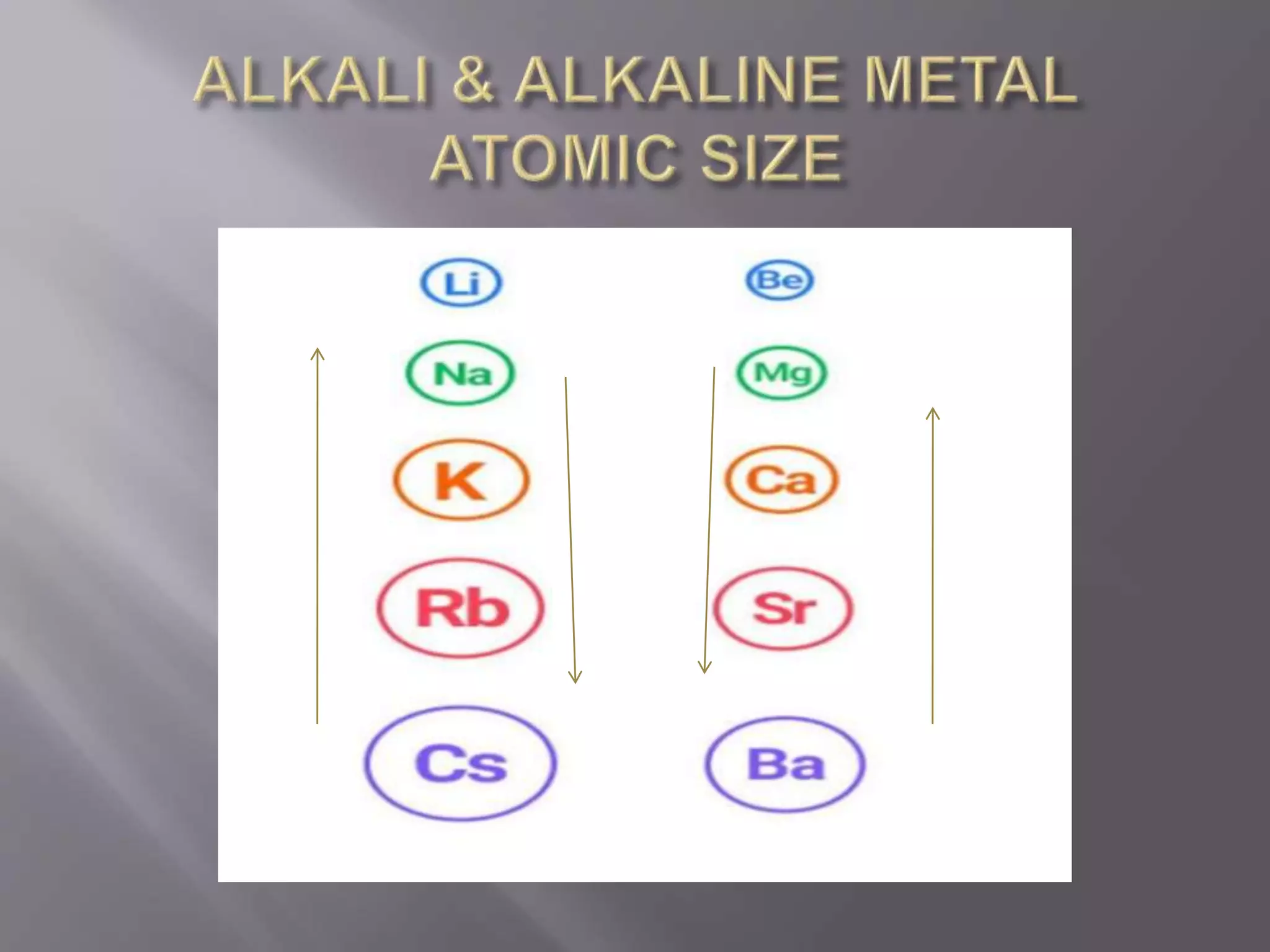
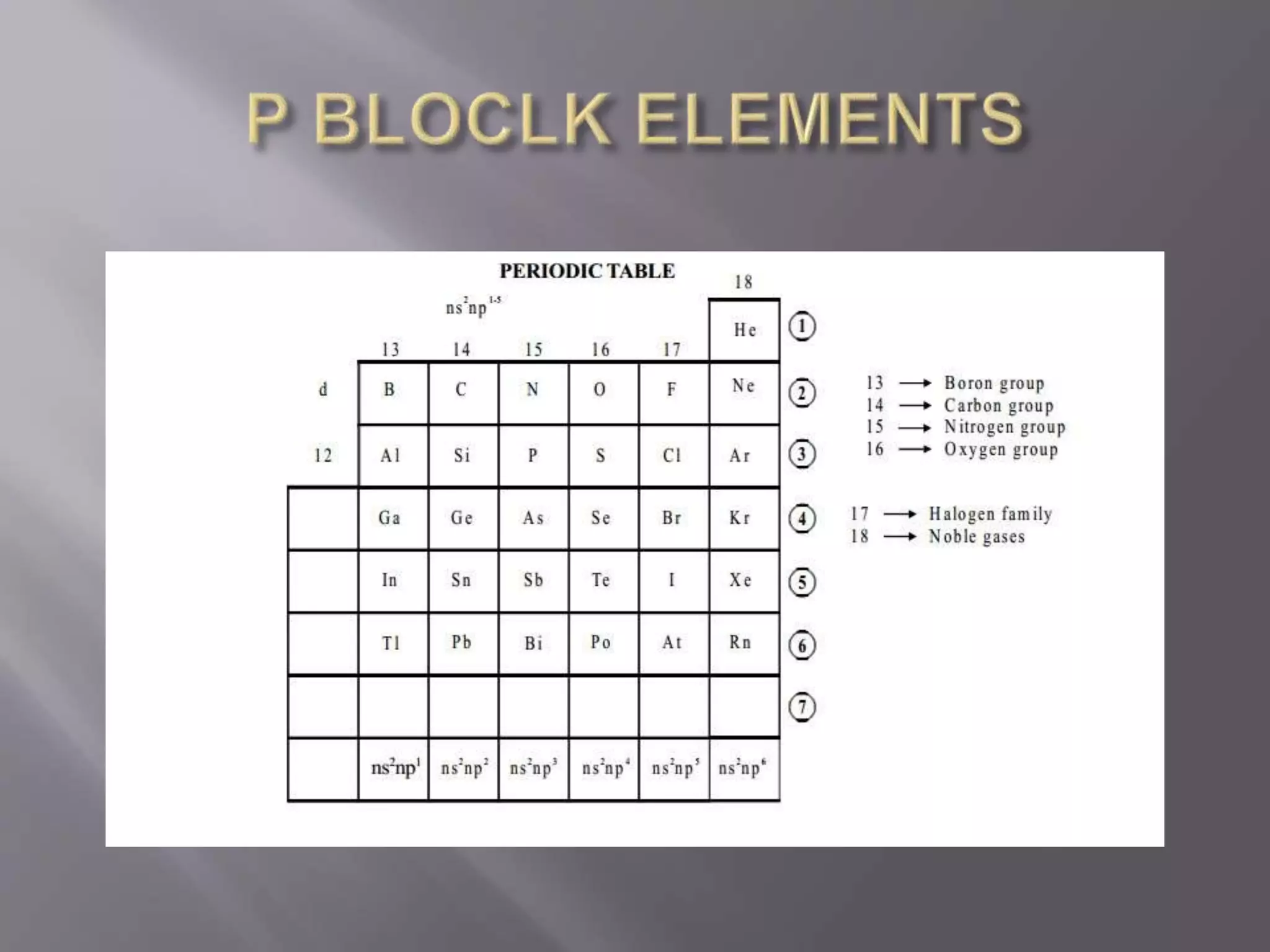
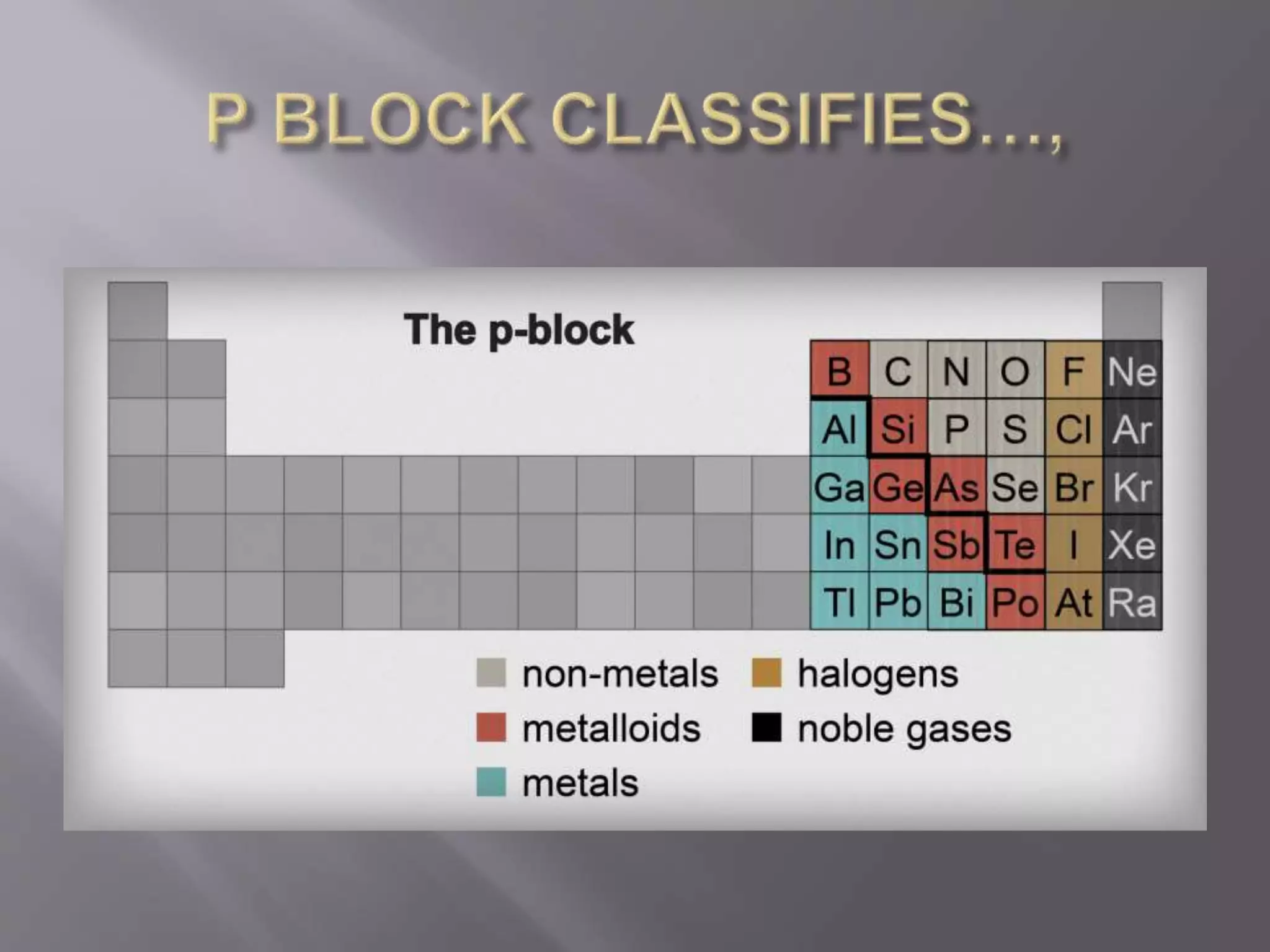

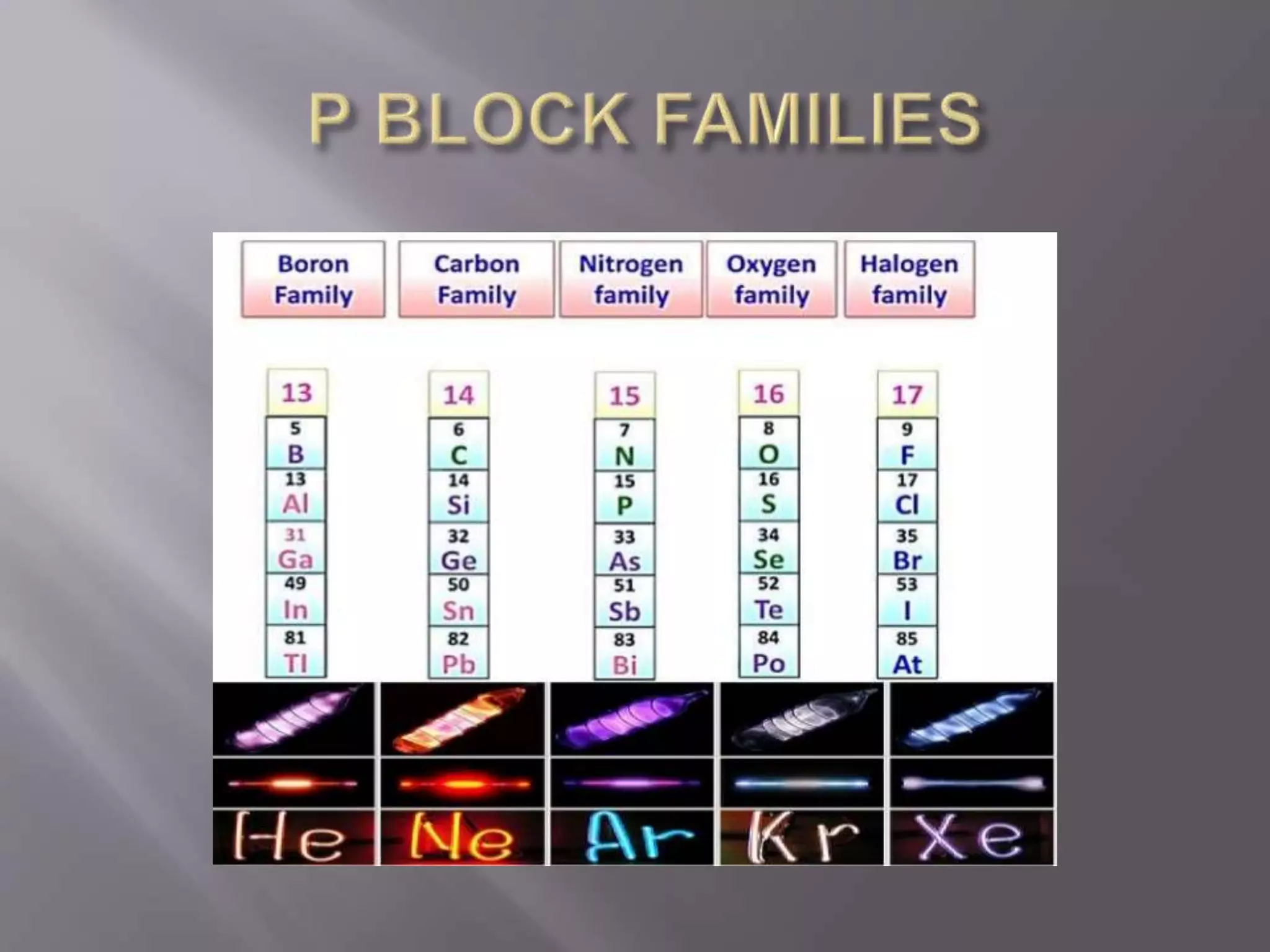
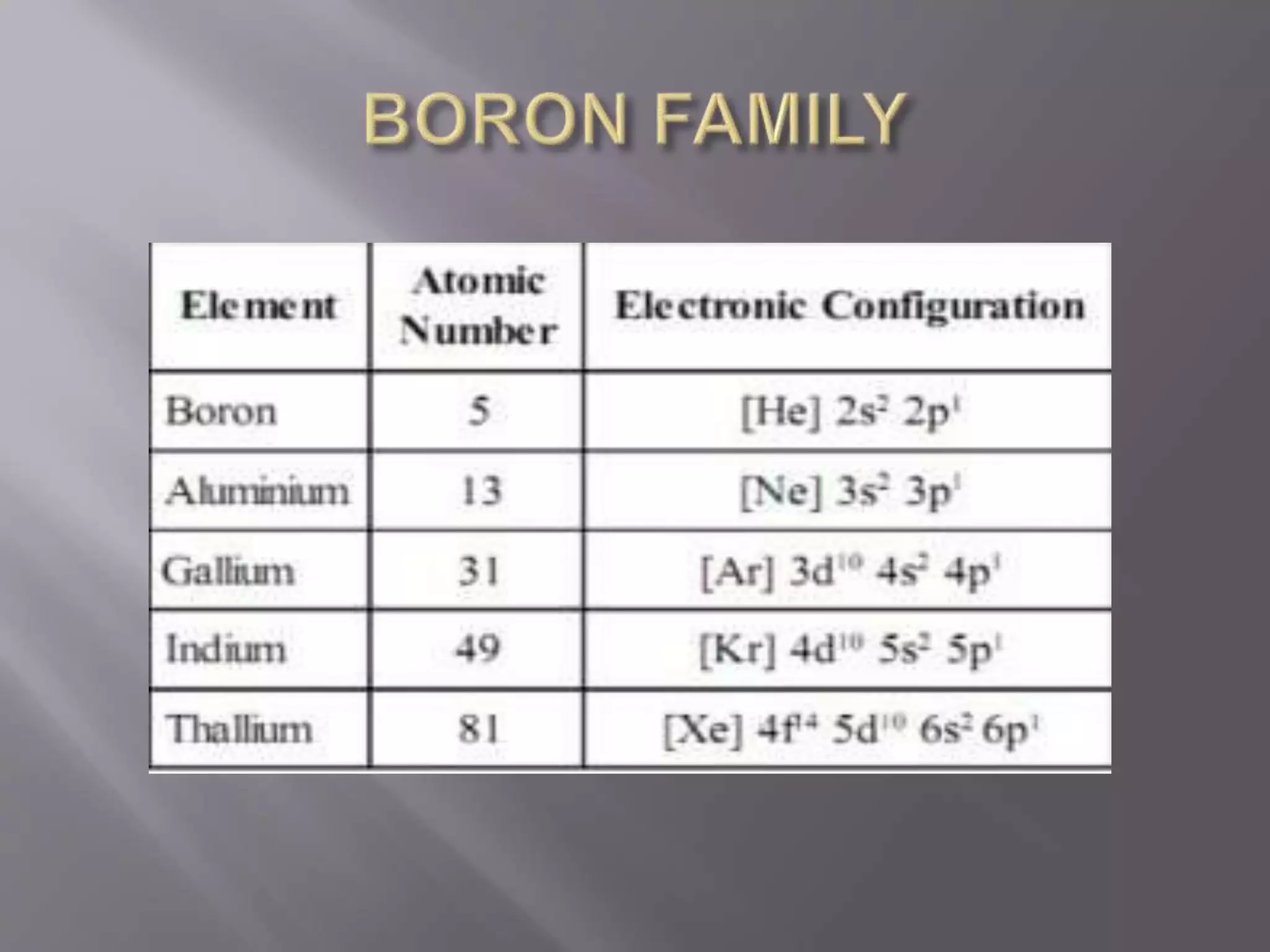
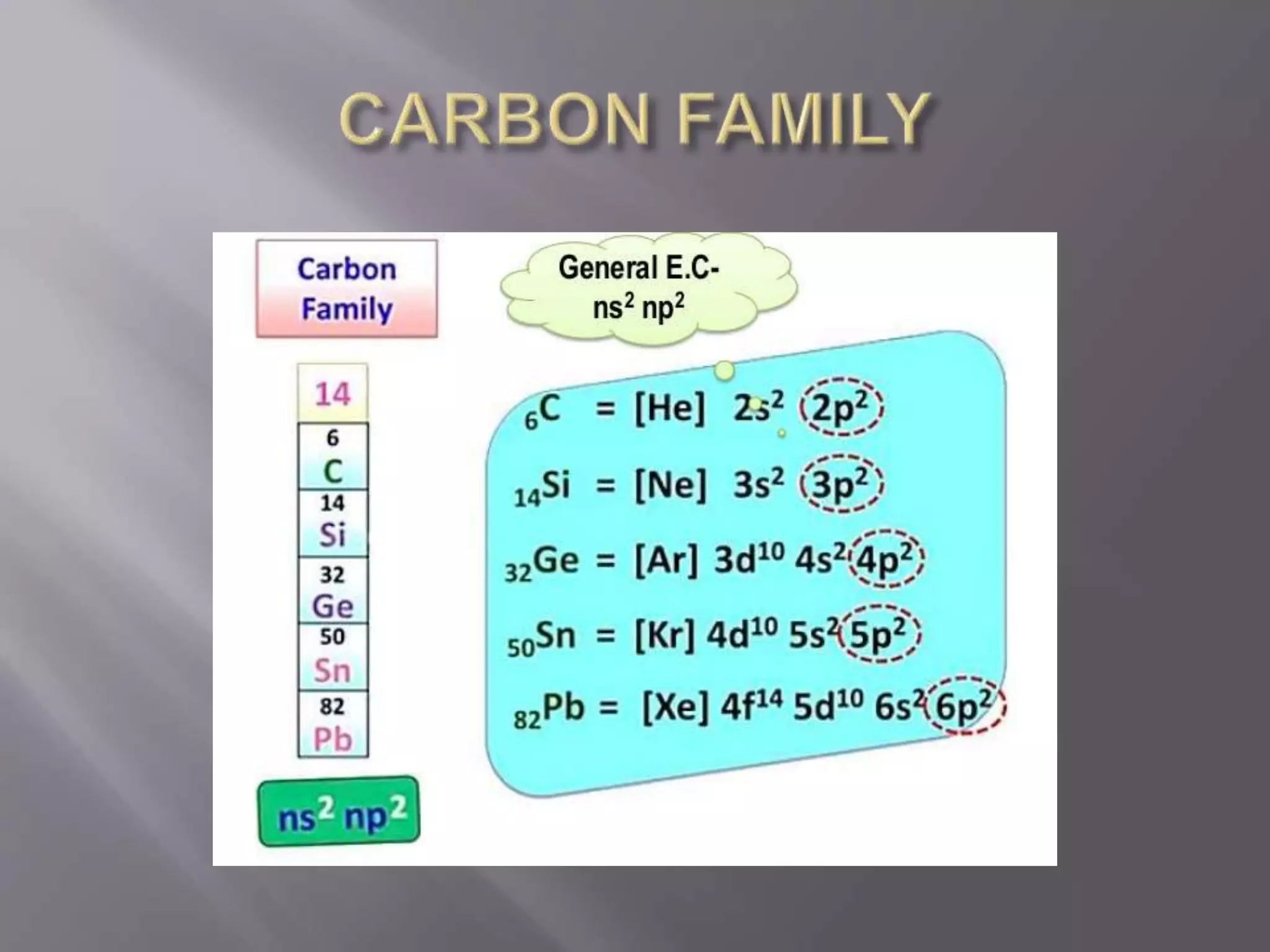
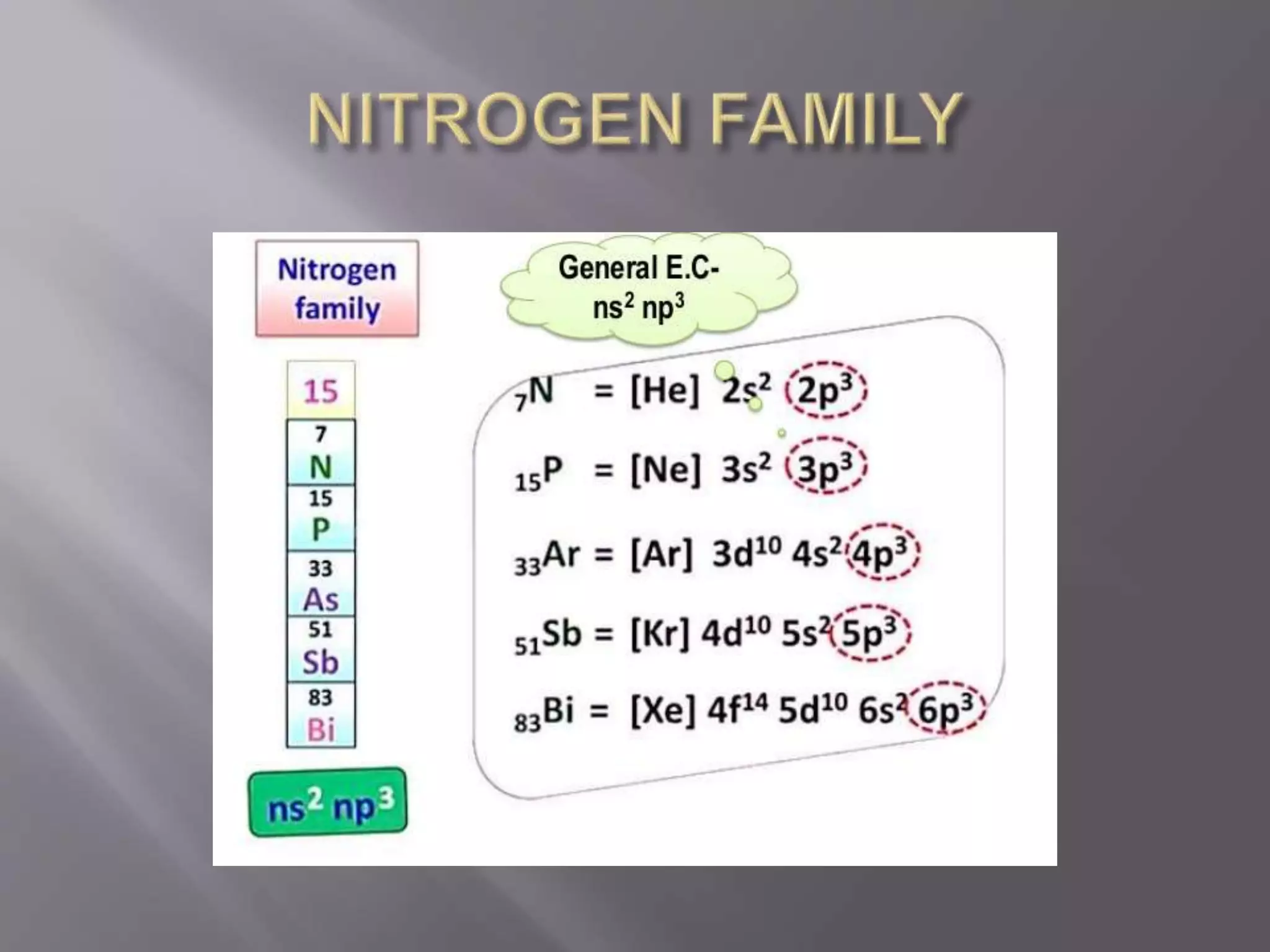
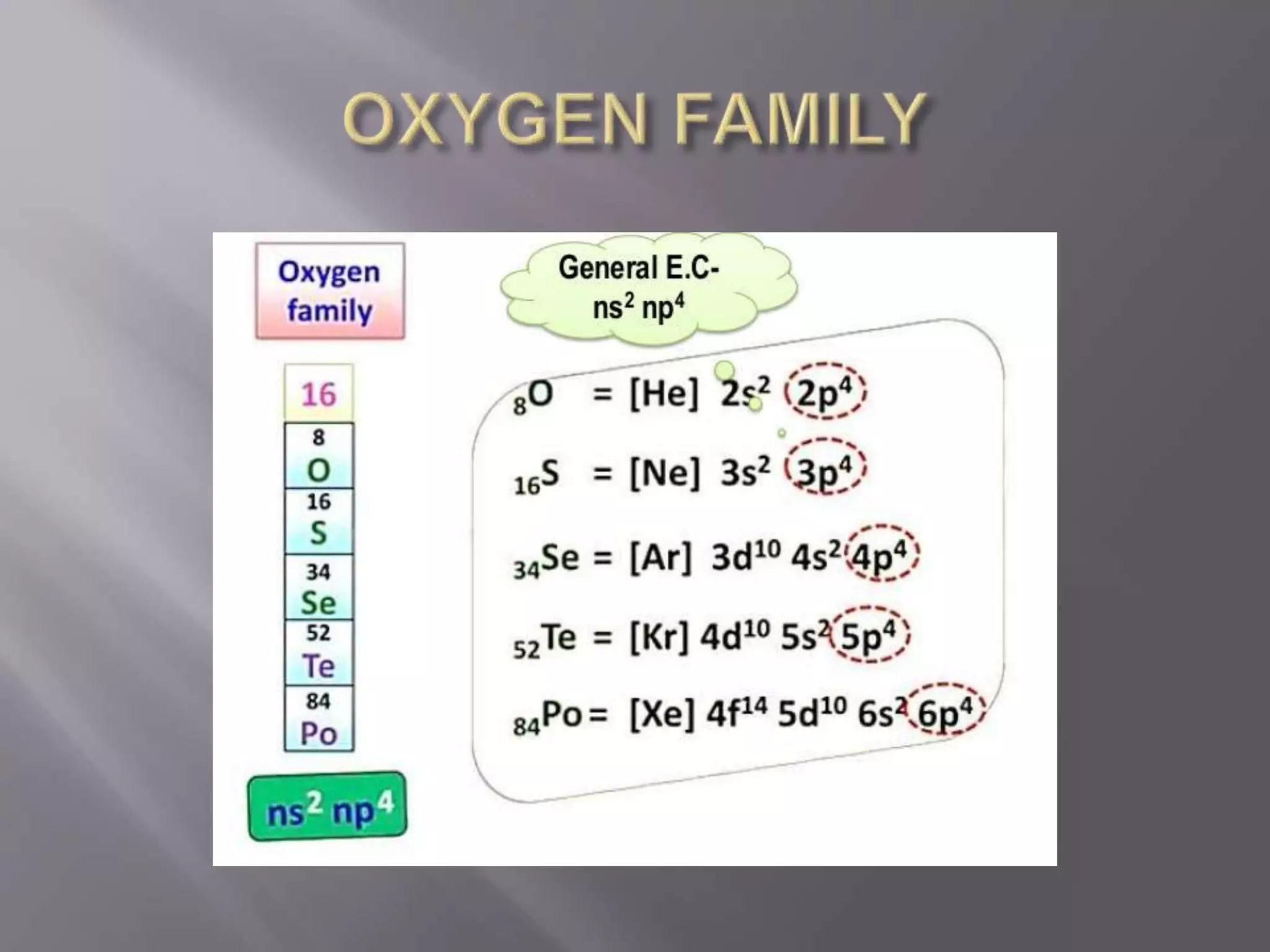
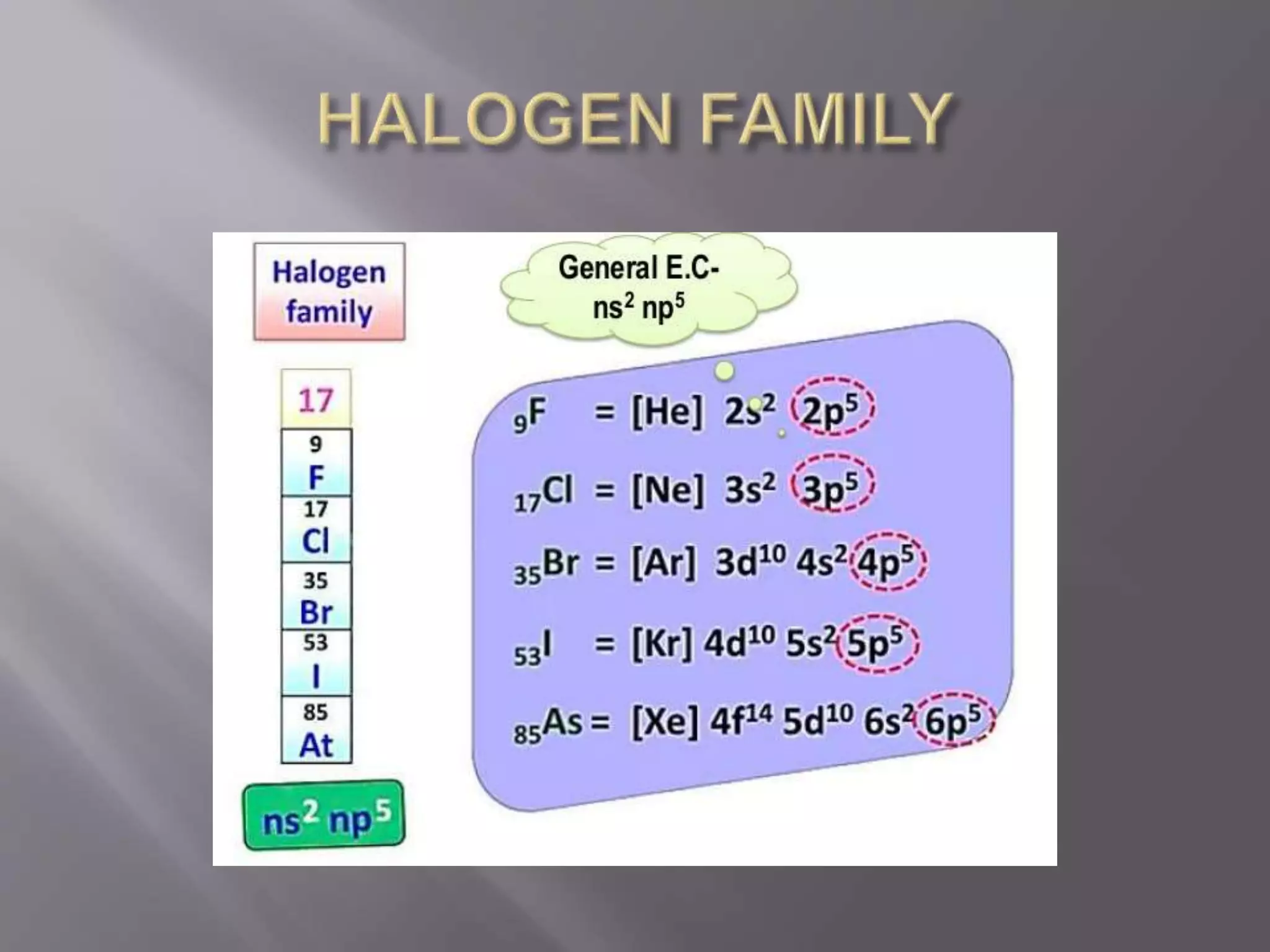
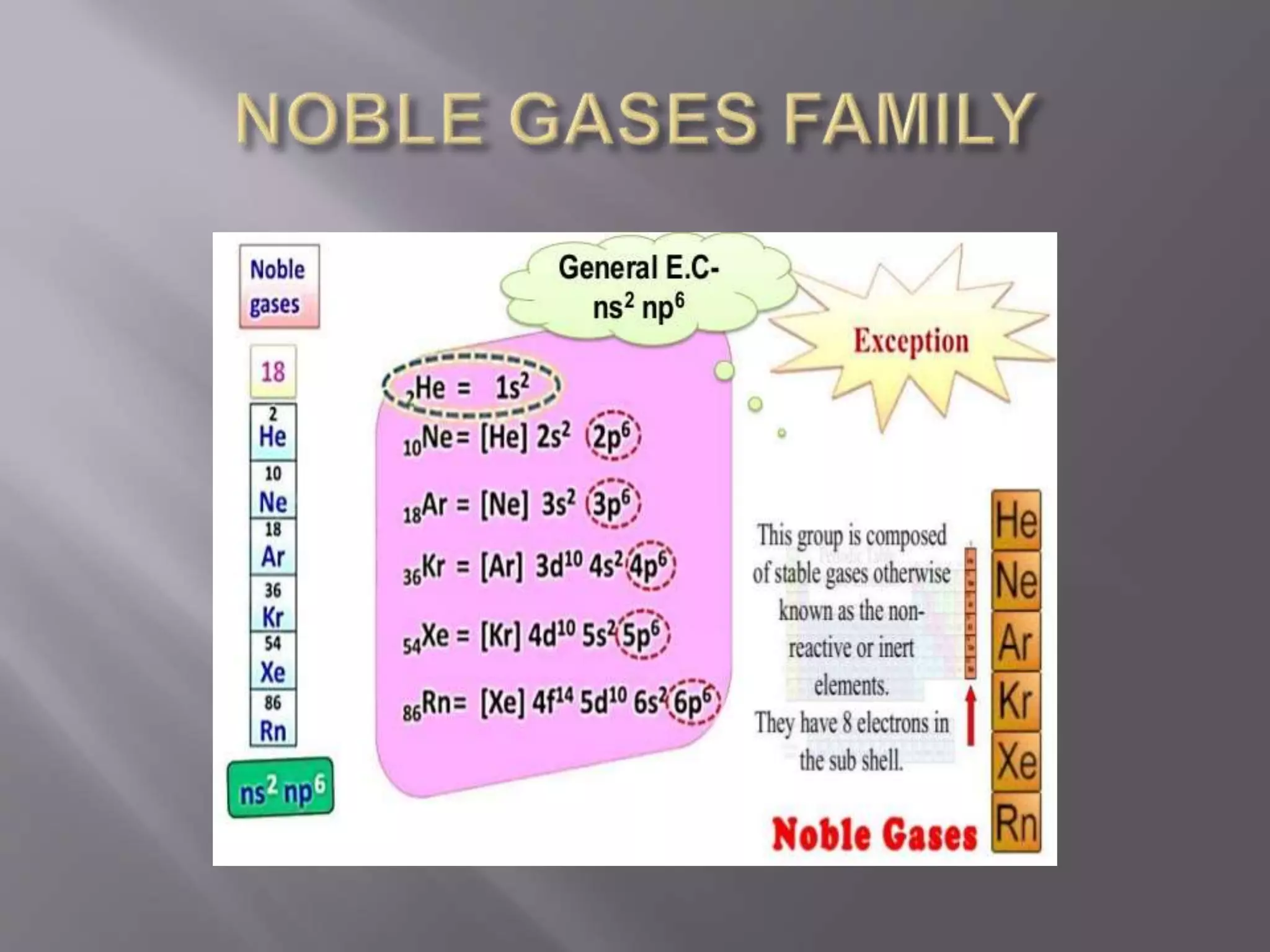
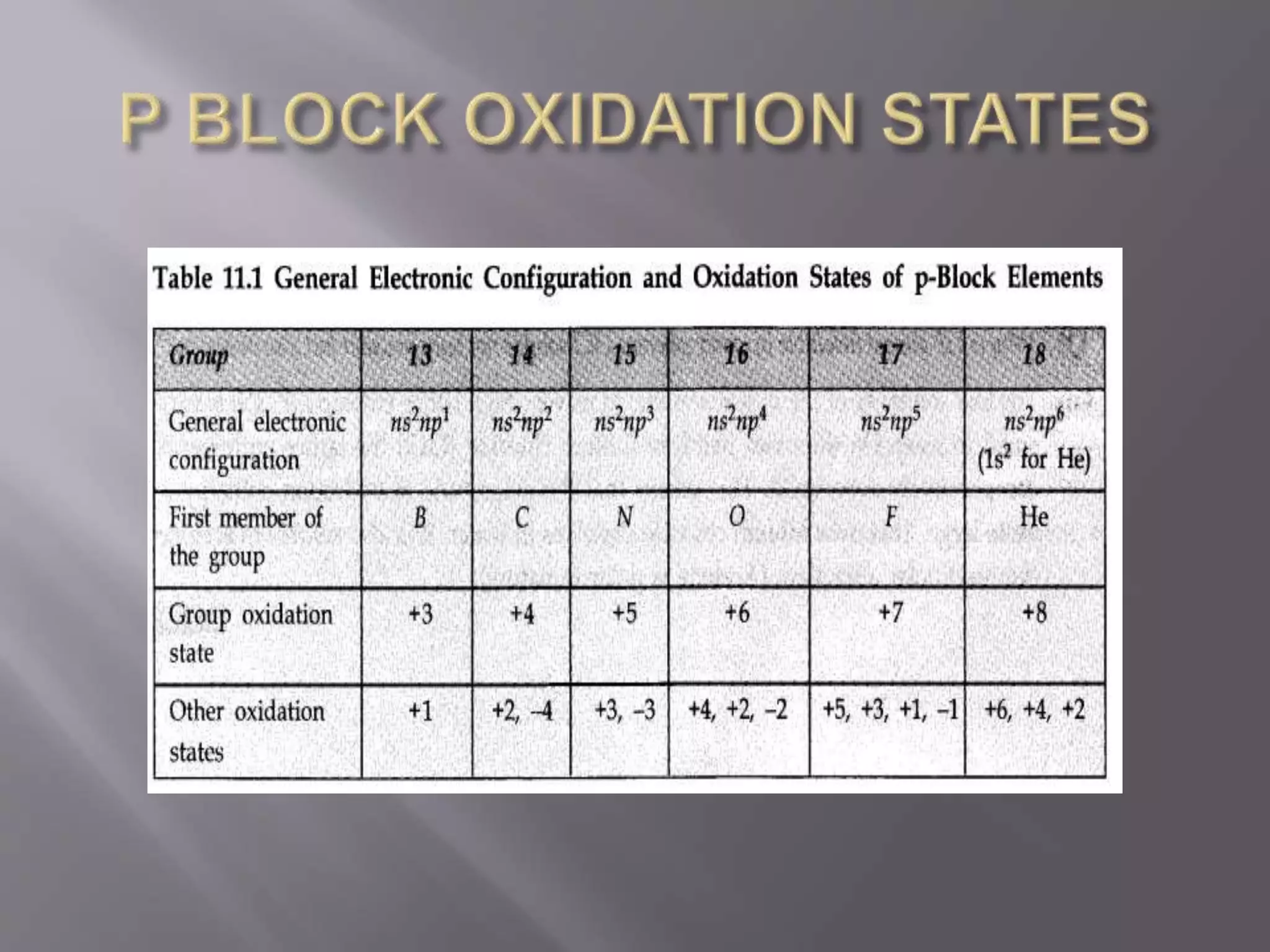

1. Main group elements, also called representative elements, consist of metals, non-metals, and metalloids. 2. These elements are classified into two main categories: alkali metals (groups IA) and alkaline earth metals (group IIA). 3. Alkali metals include lithium, sodium, potassium, rubidium, cesium, and francium and have an ns1 electronic configuration. Alkaline earth metals include beryllium, magnesium, calcium, strontium, barium, and radium and have an ns2 electronic configuration.




















