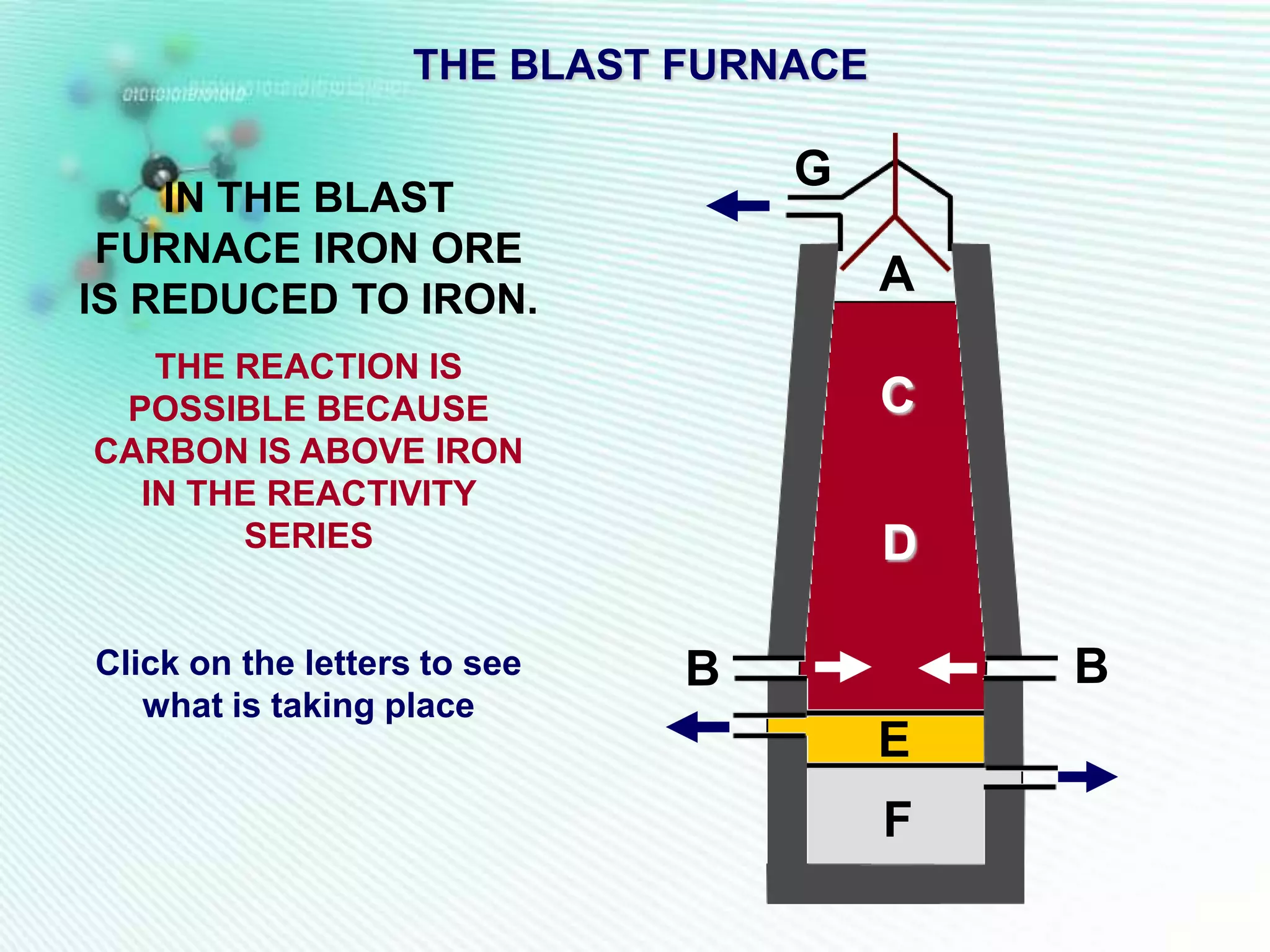
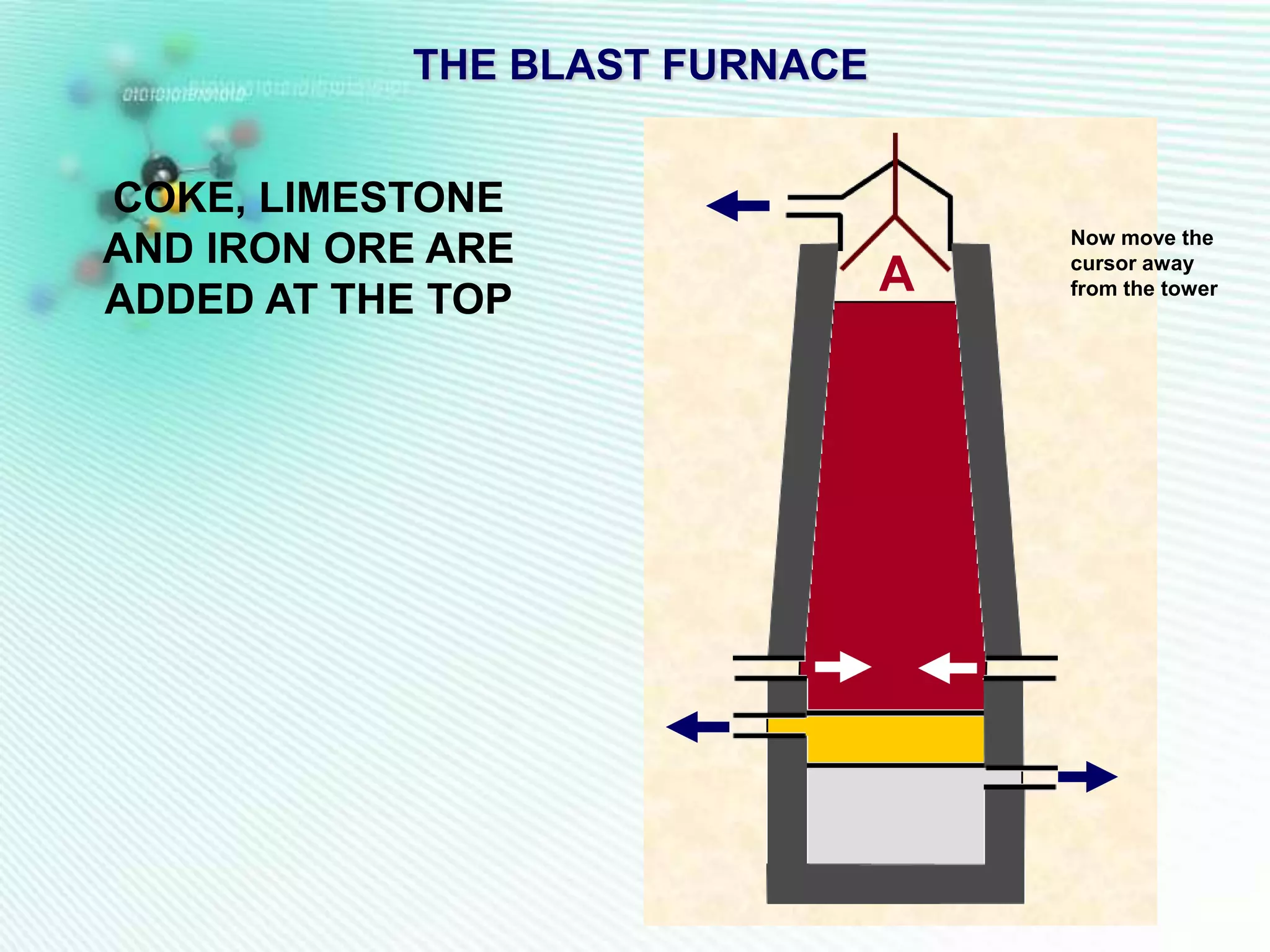
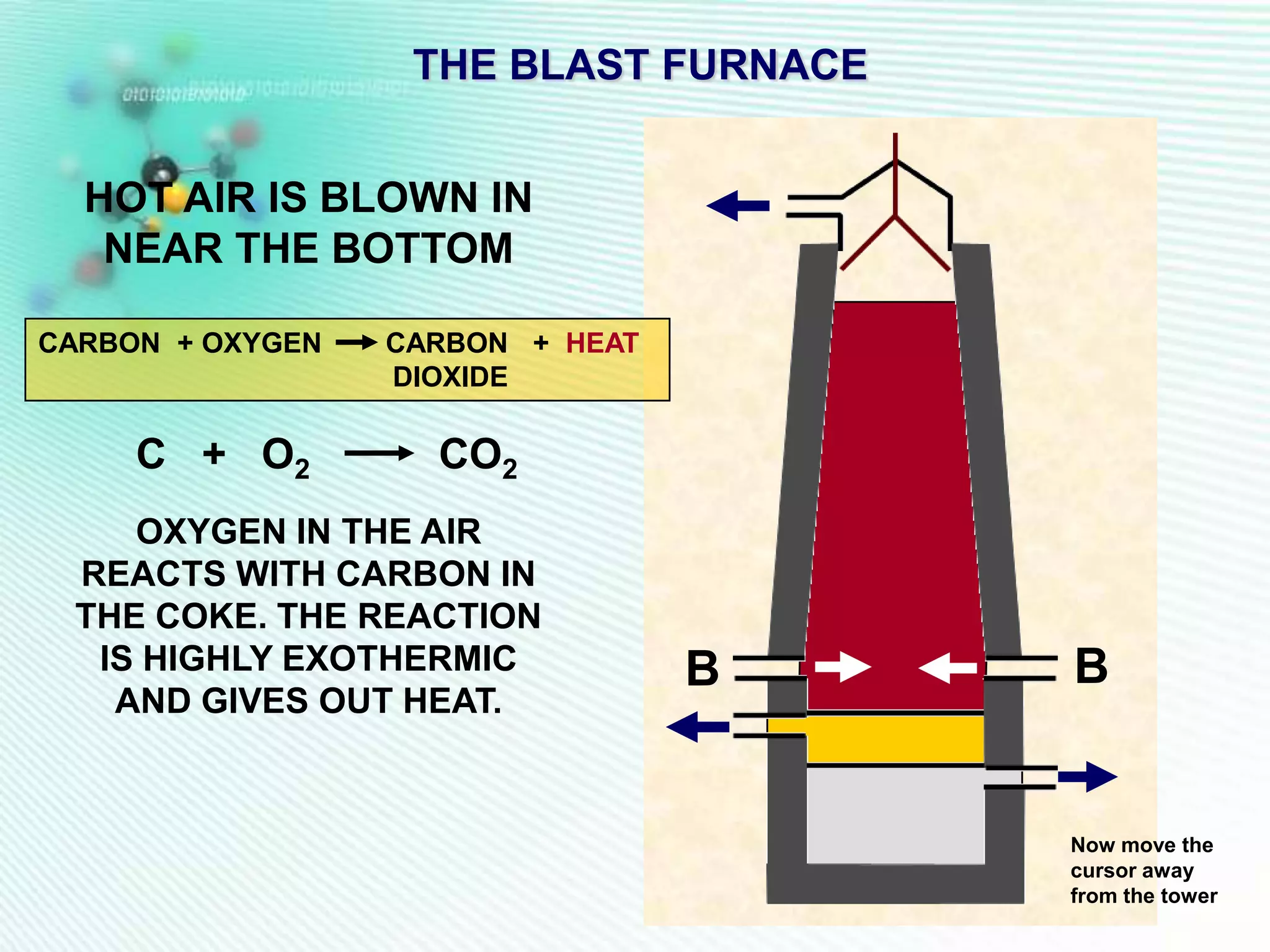
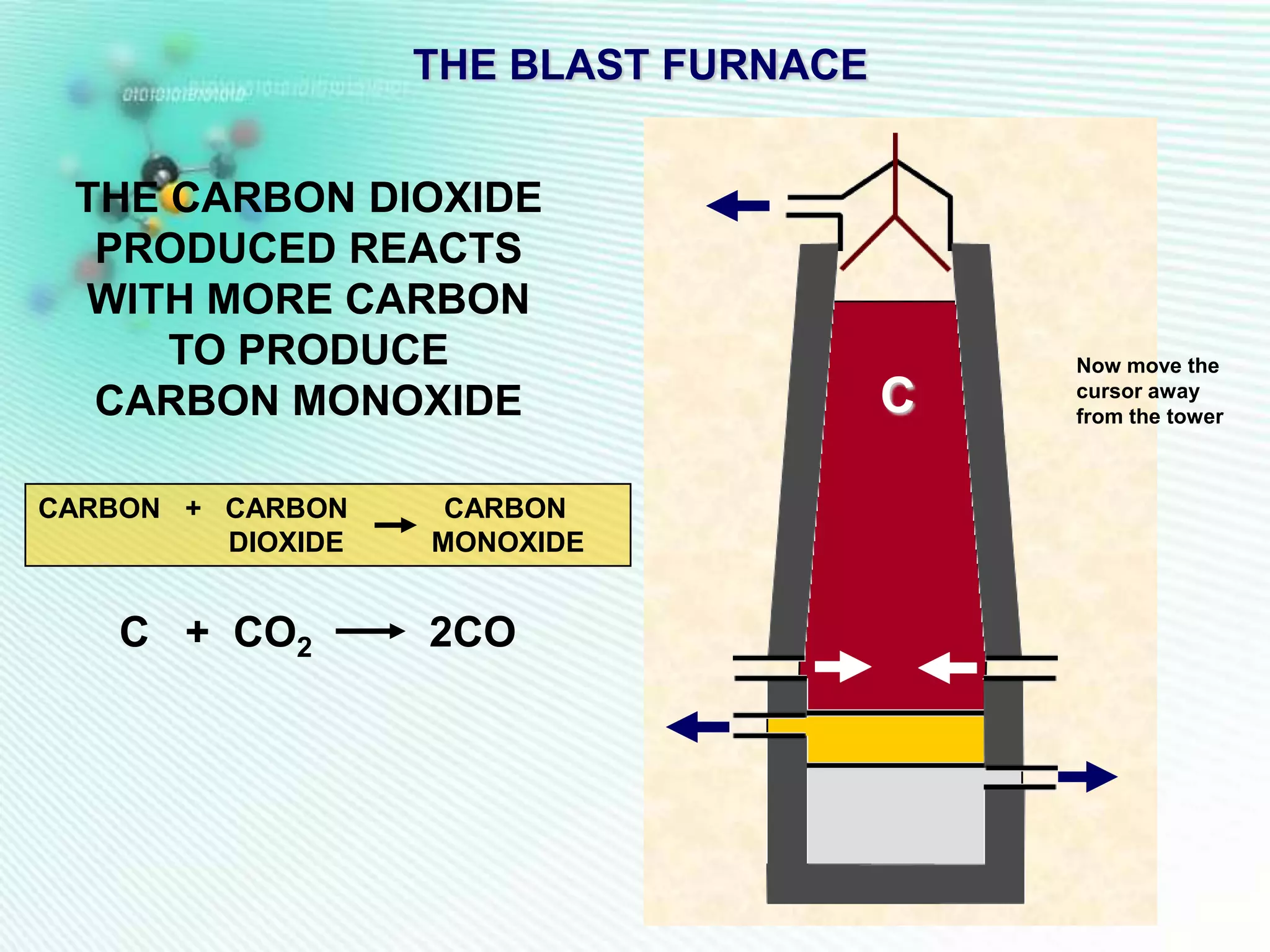
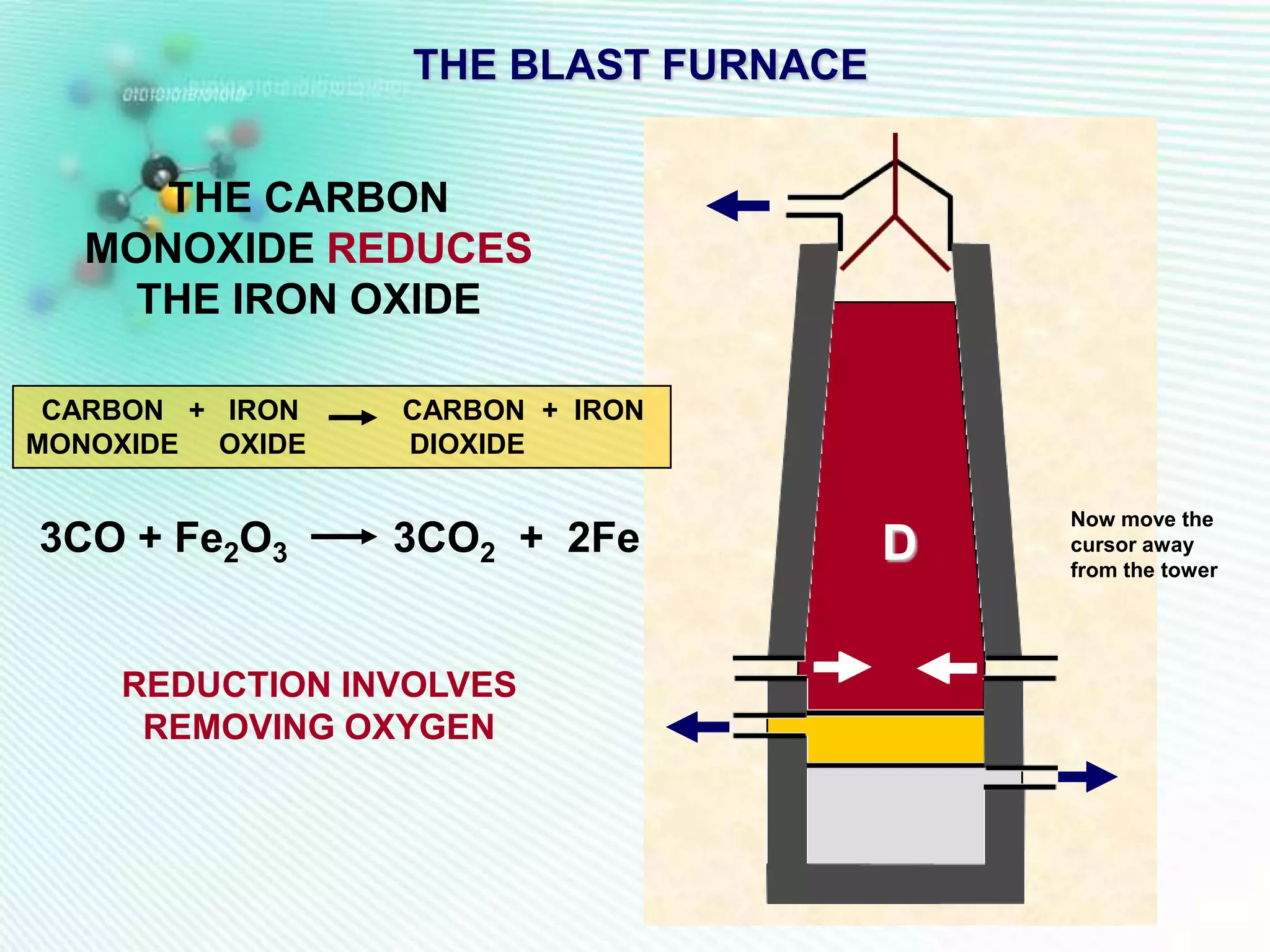
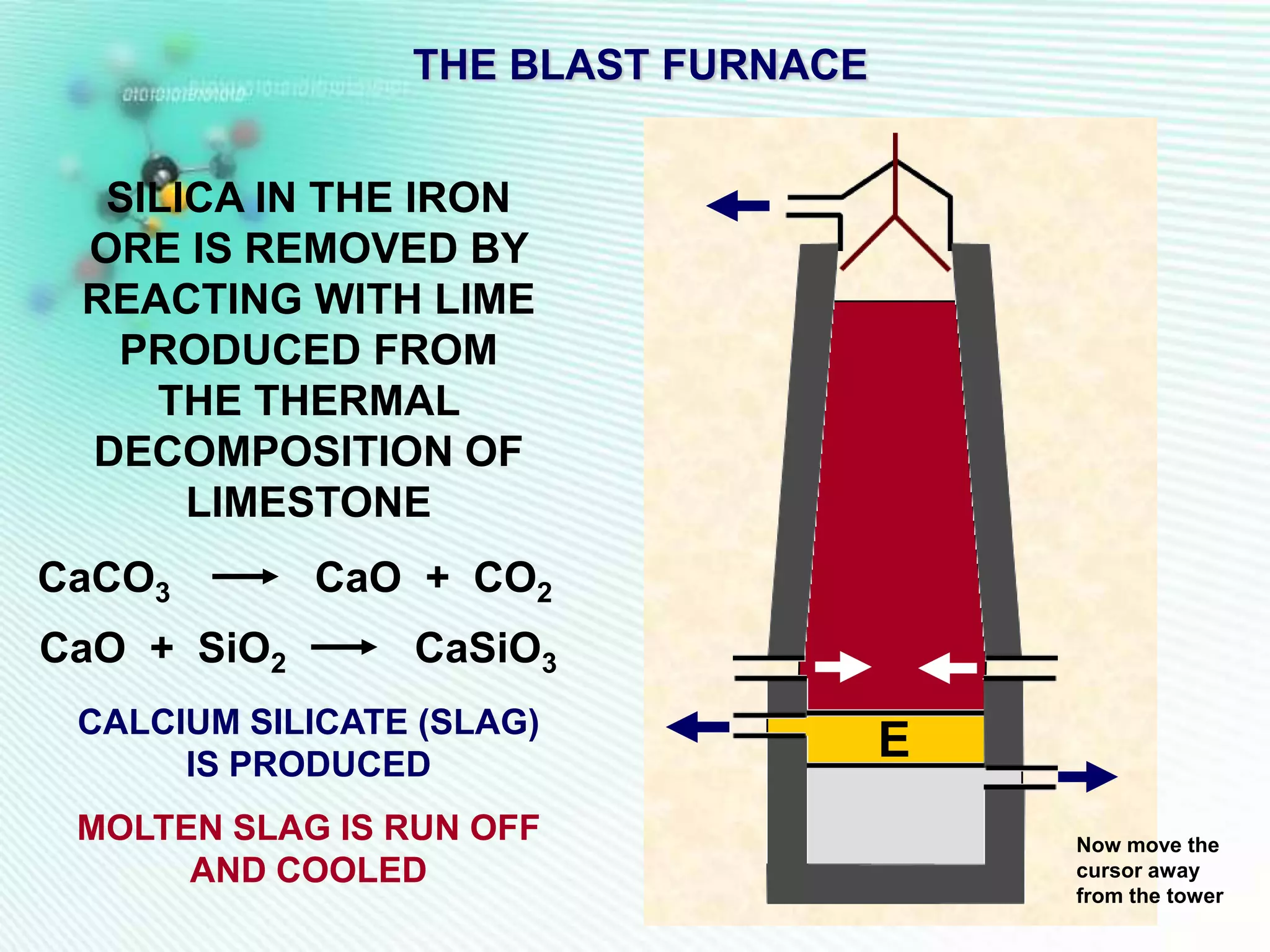
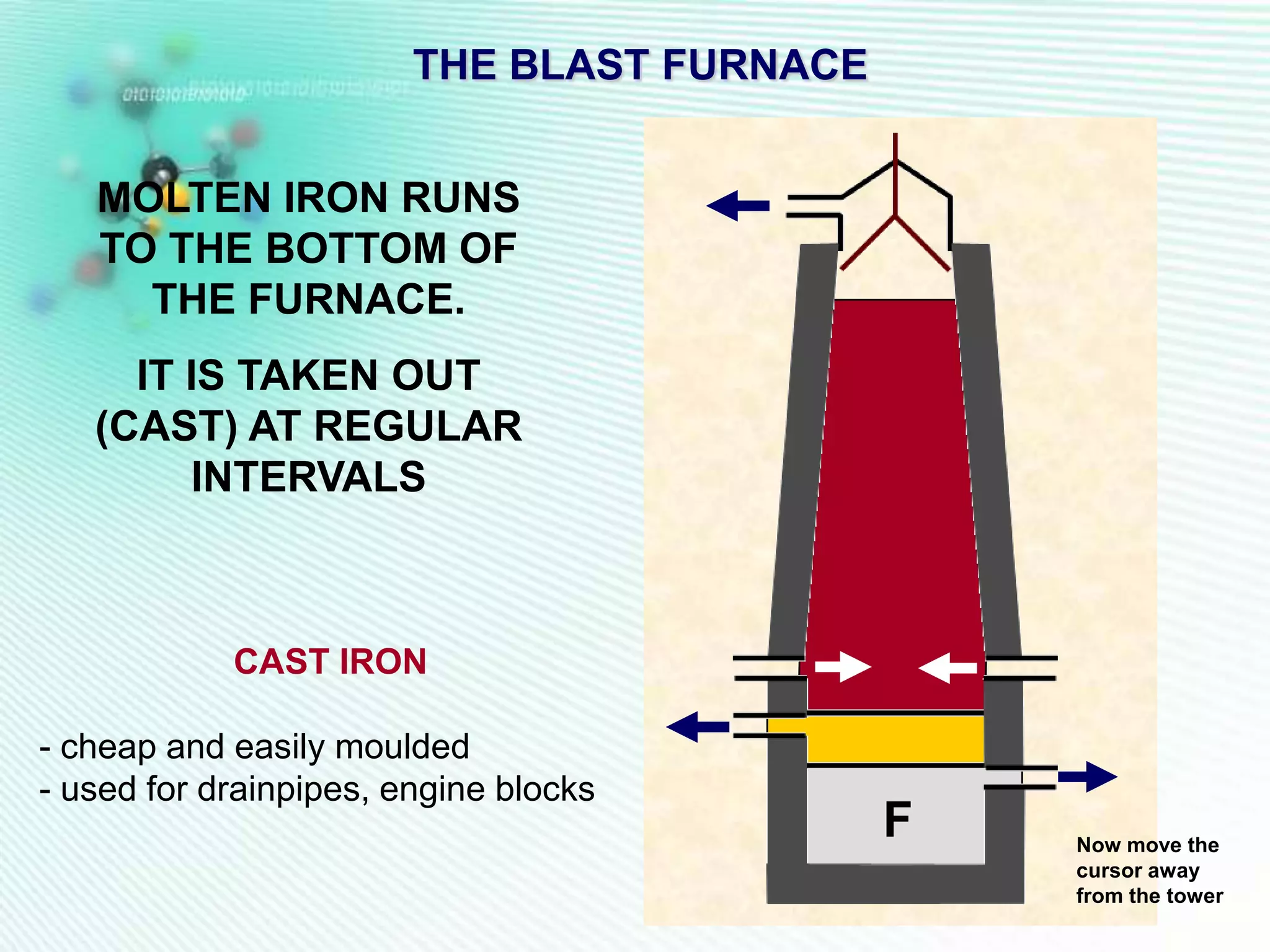
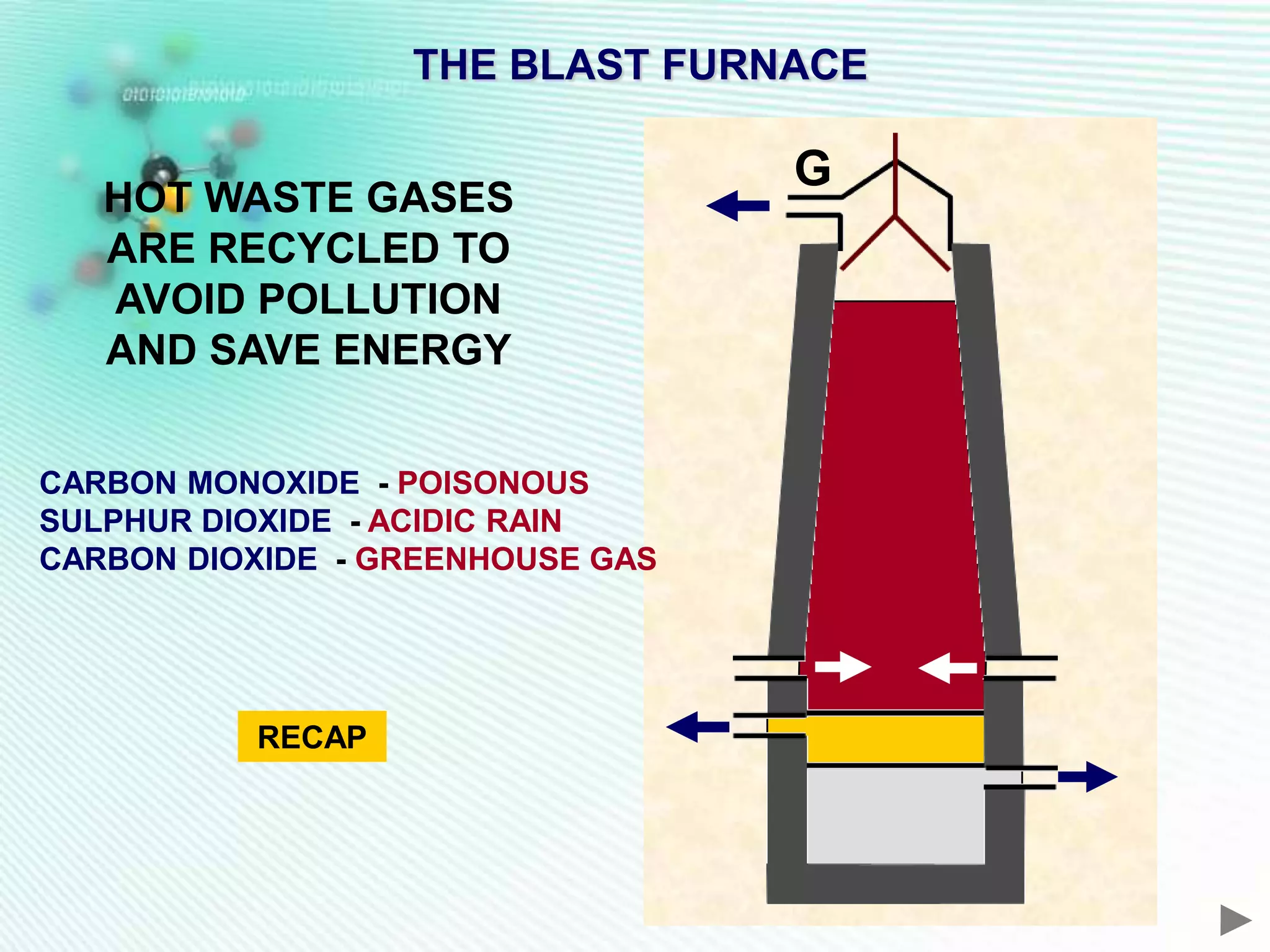
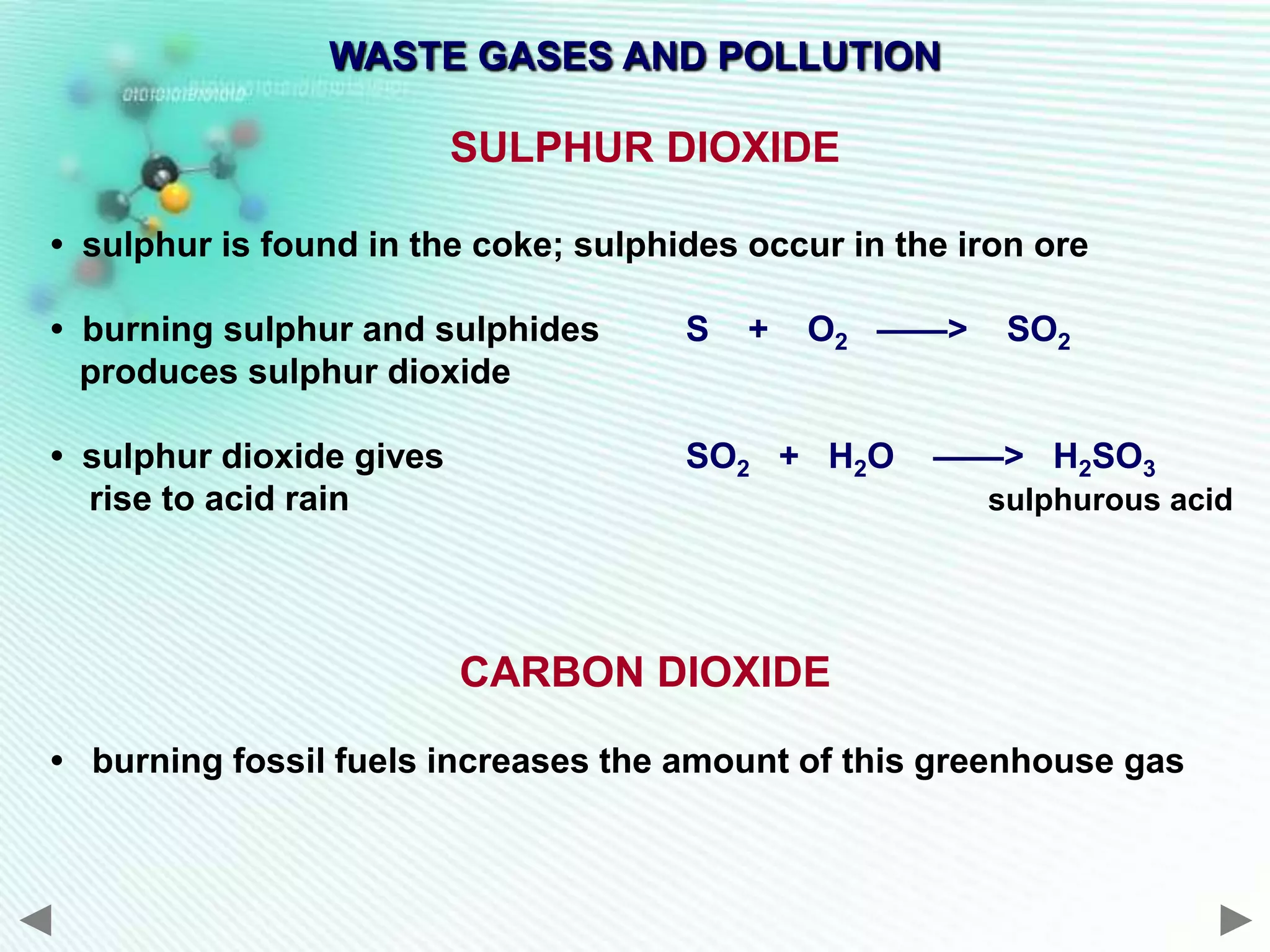
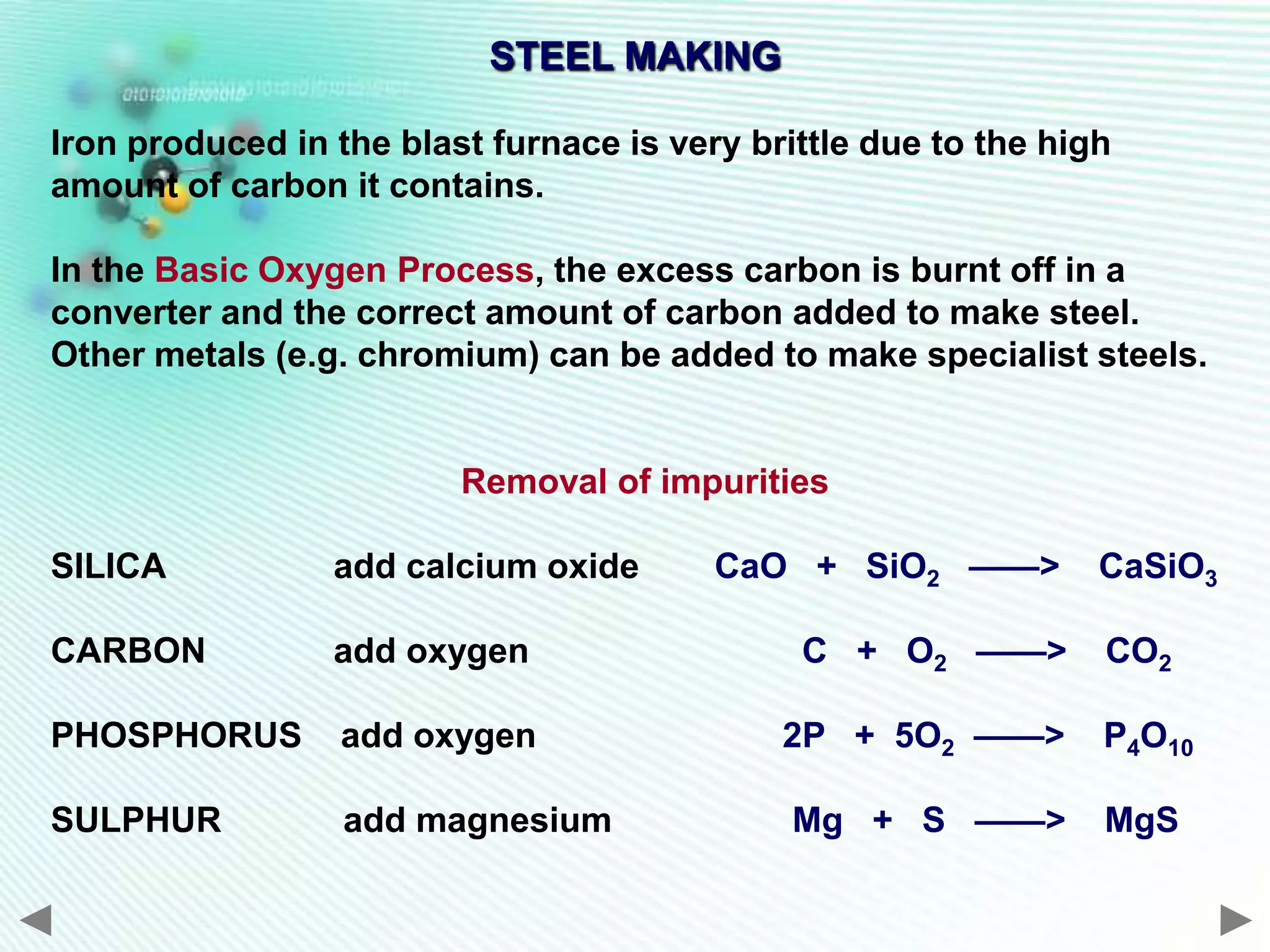
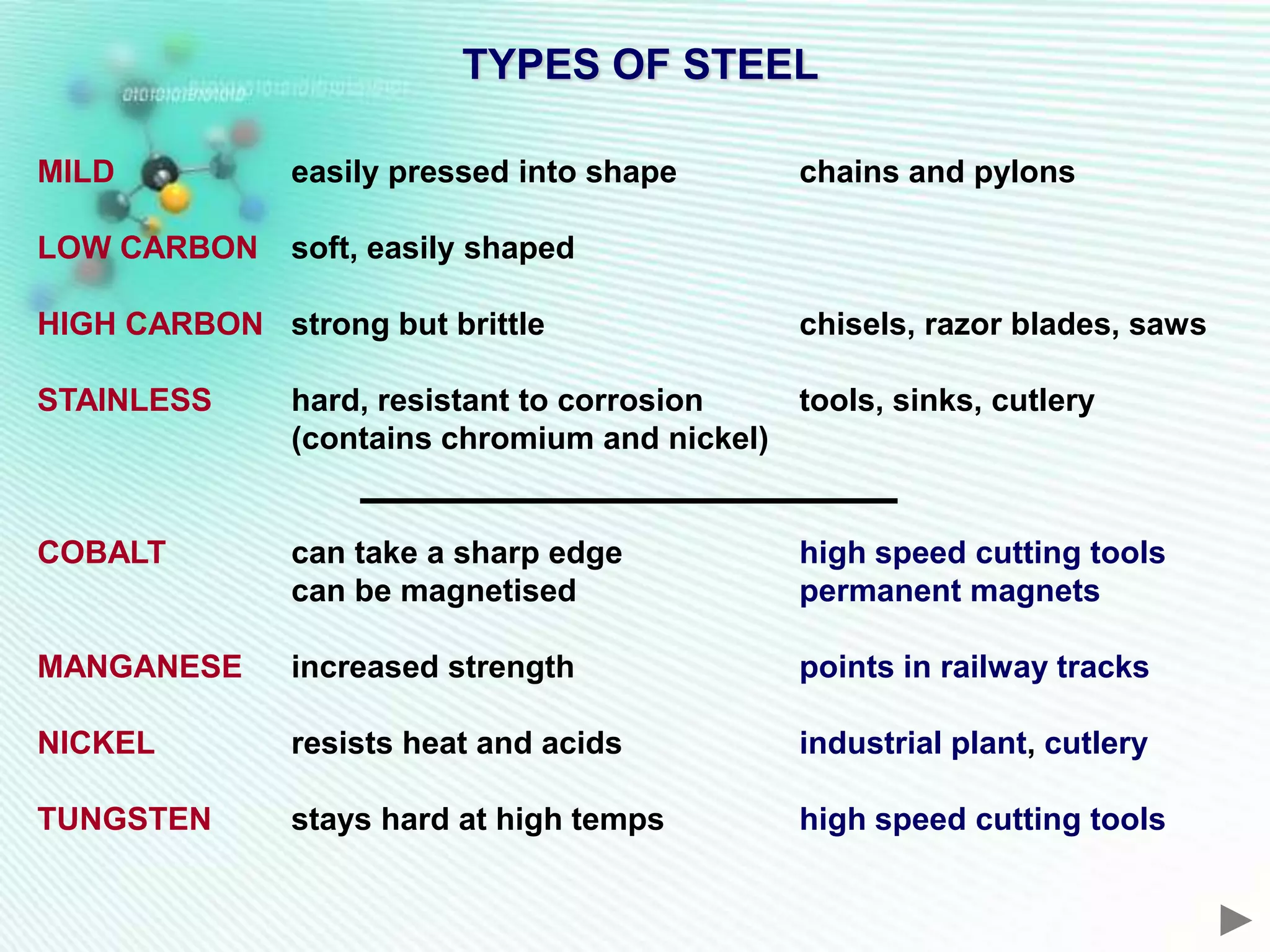
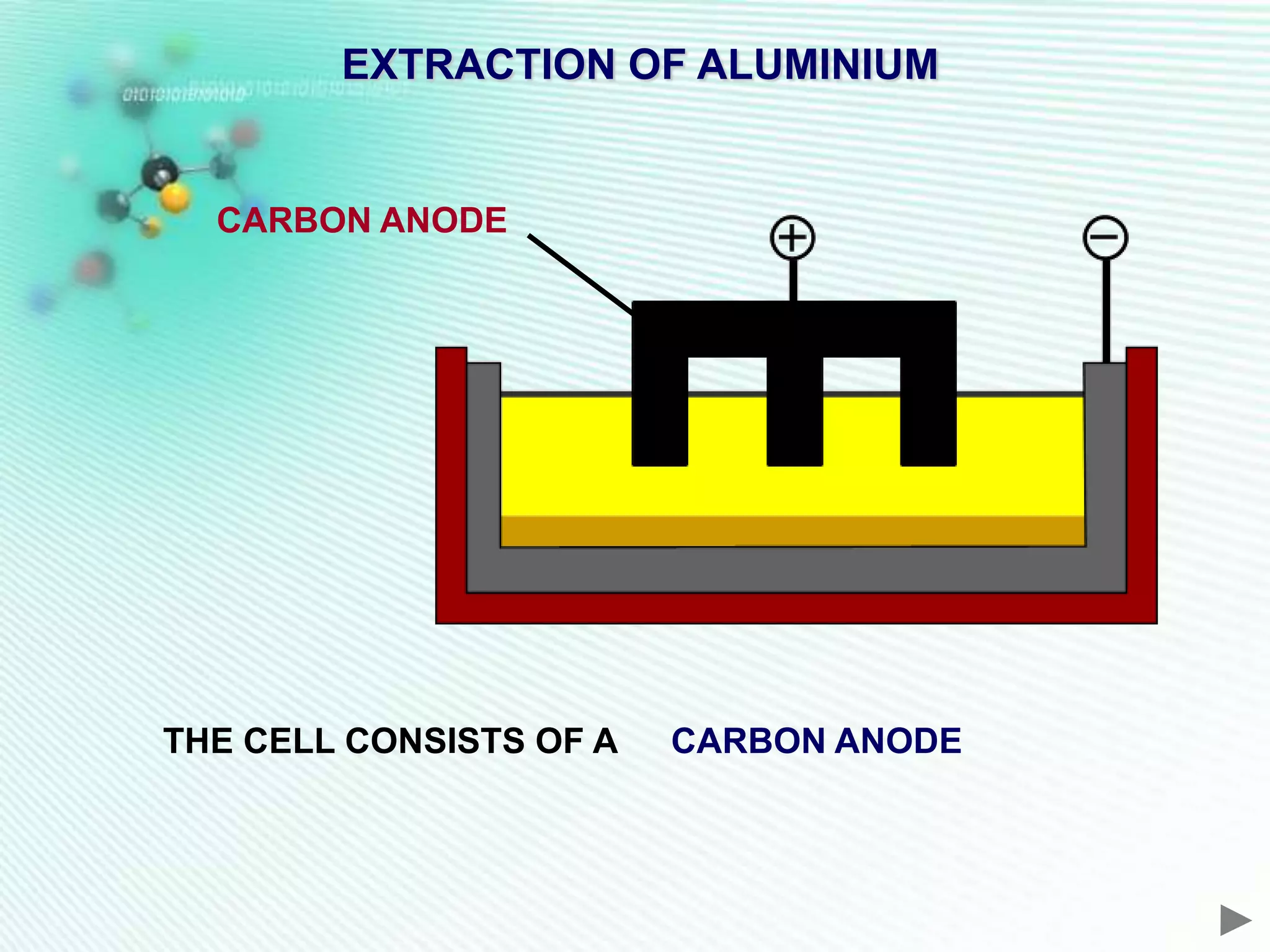
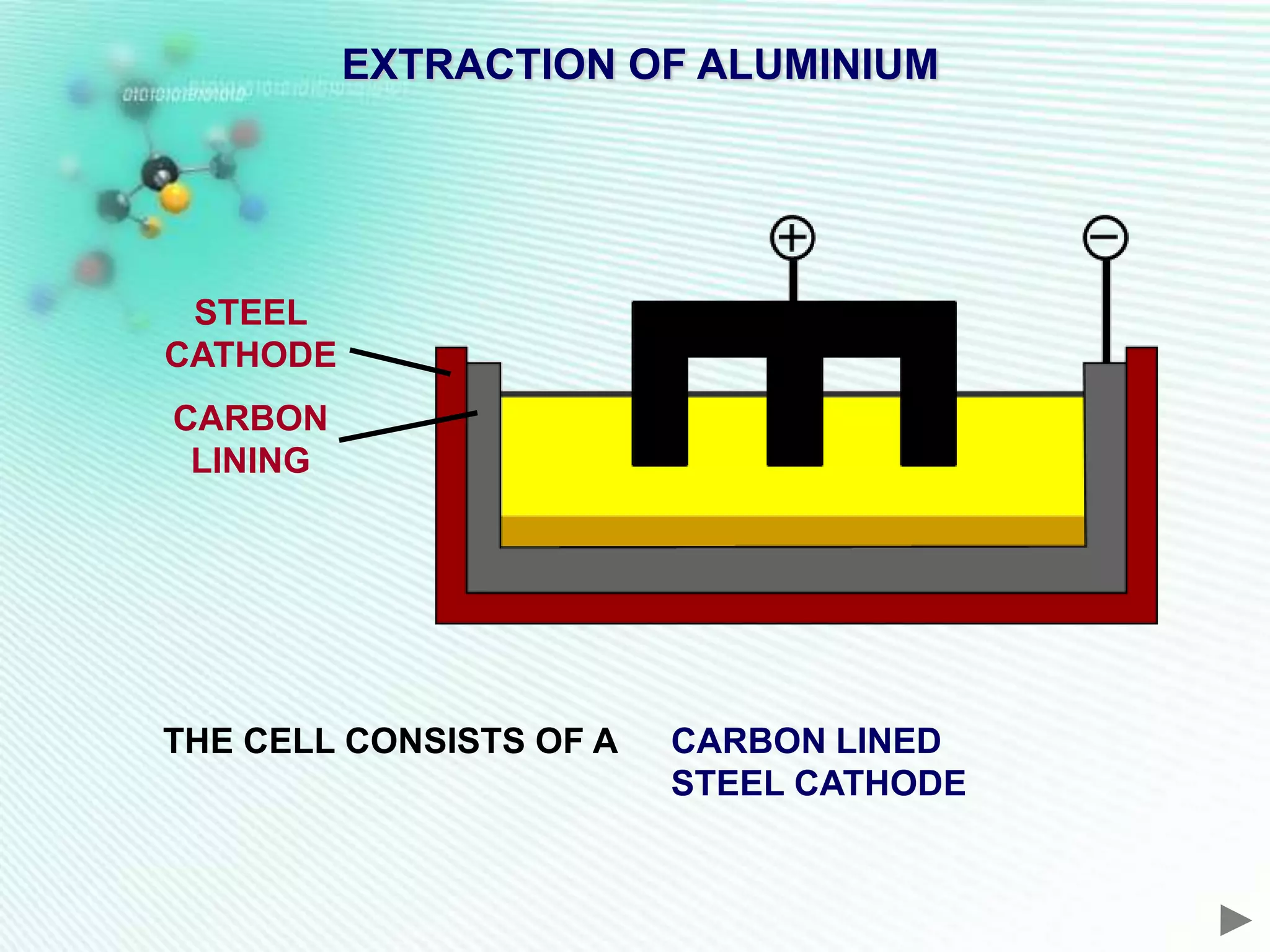
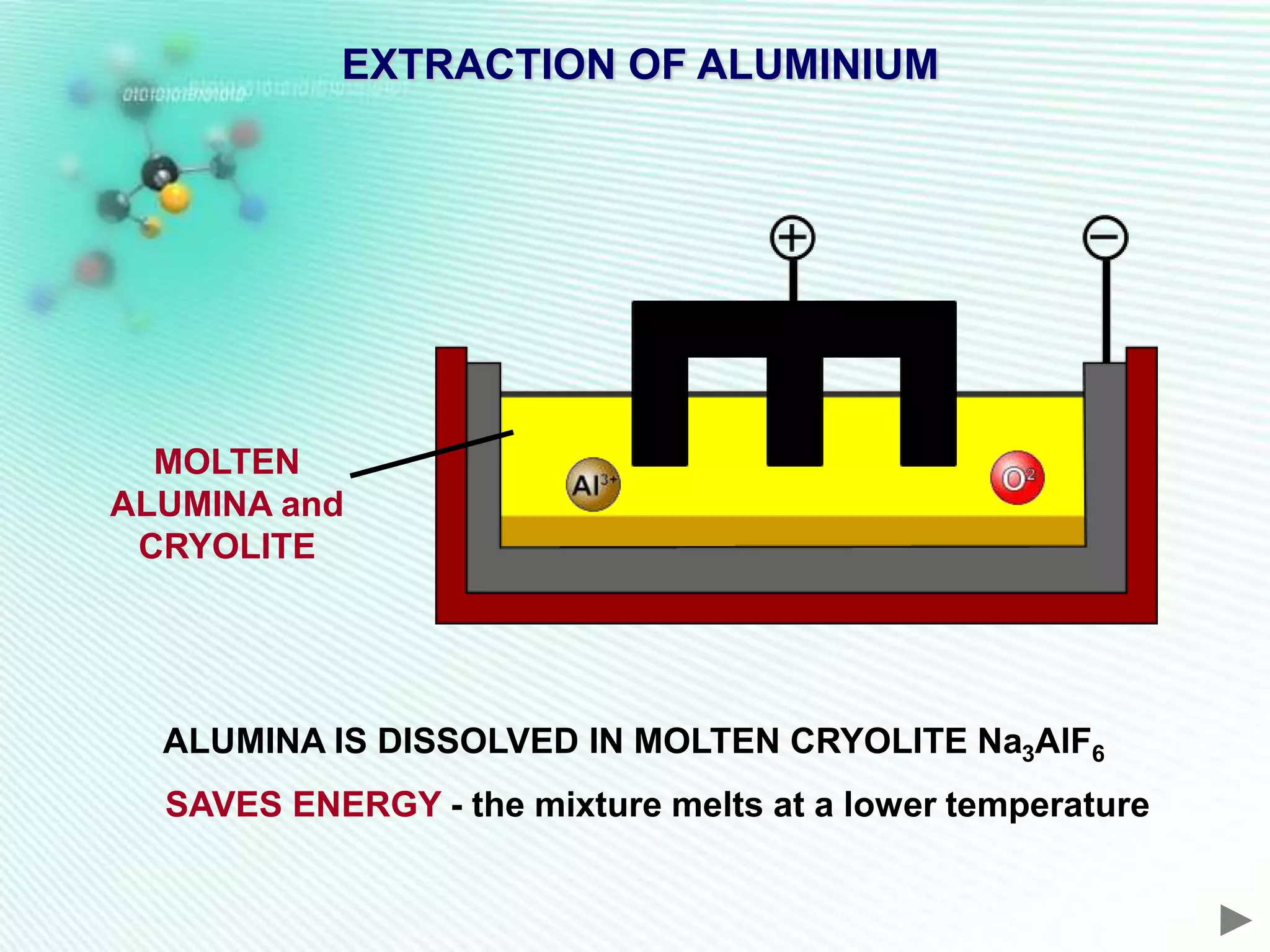
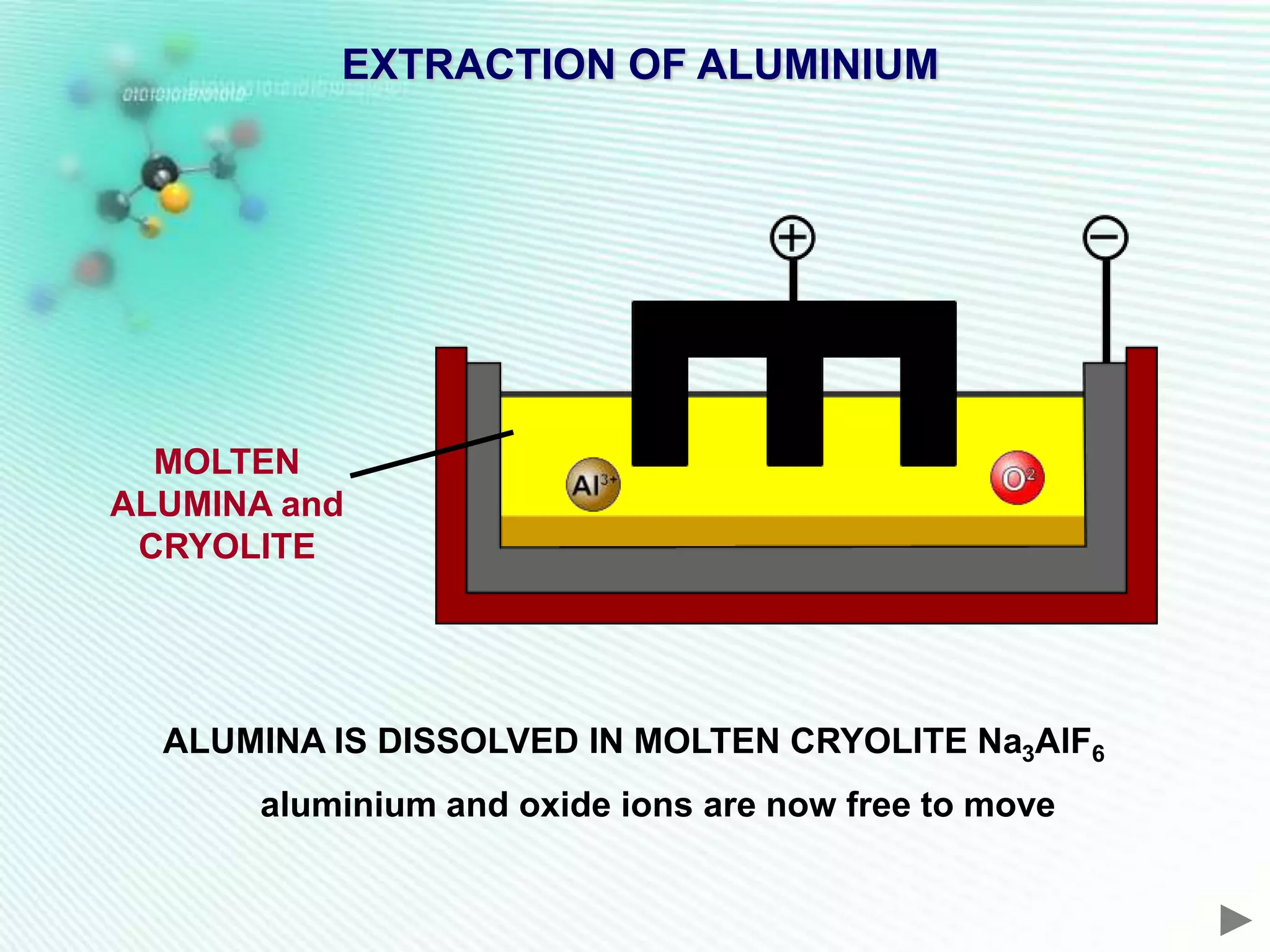
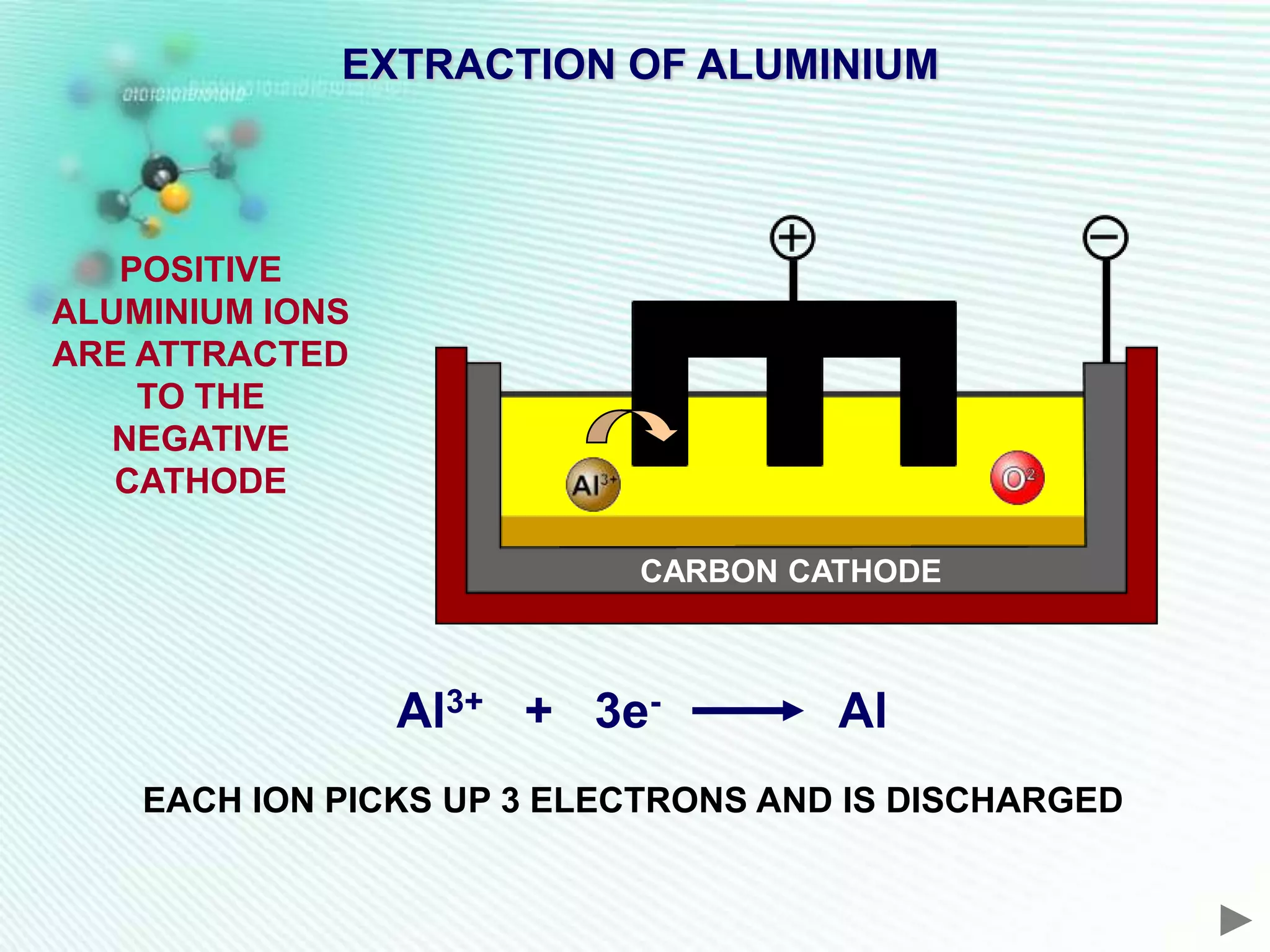
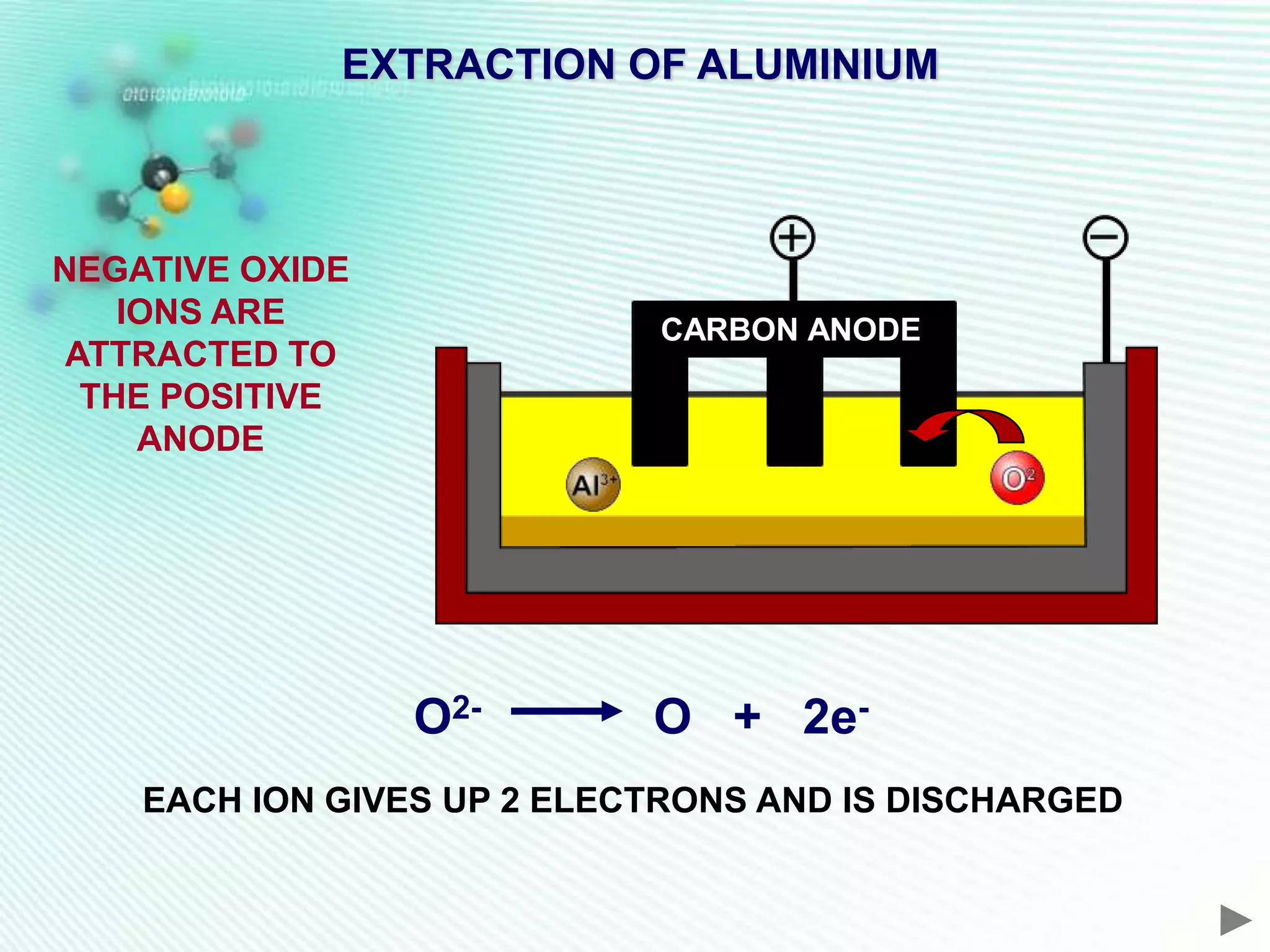
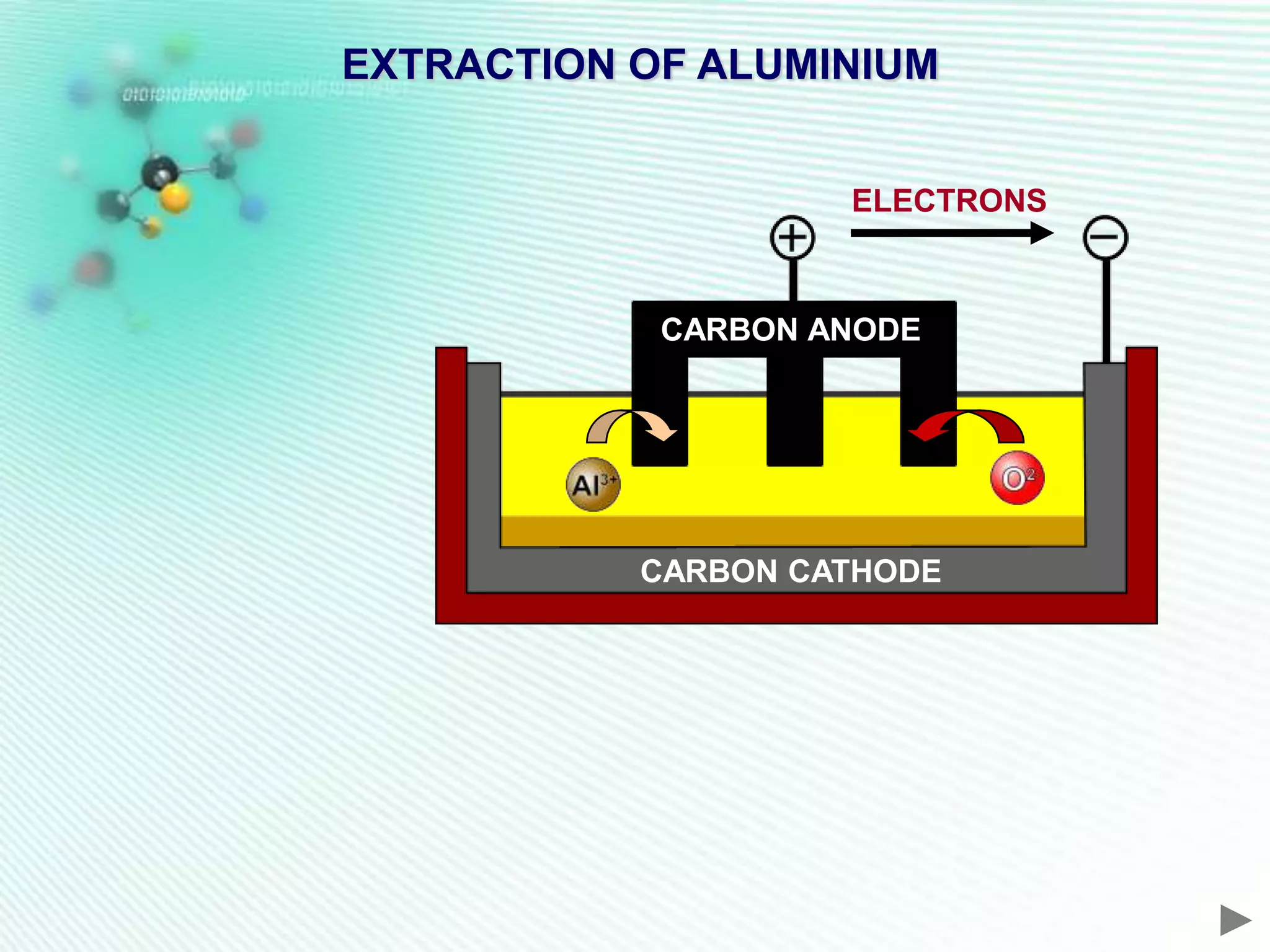
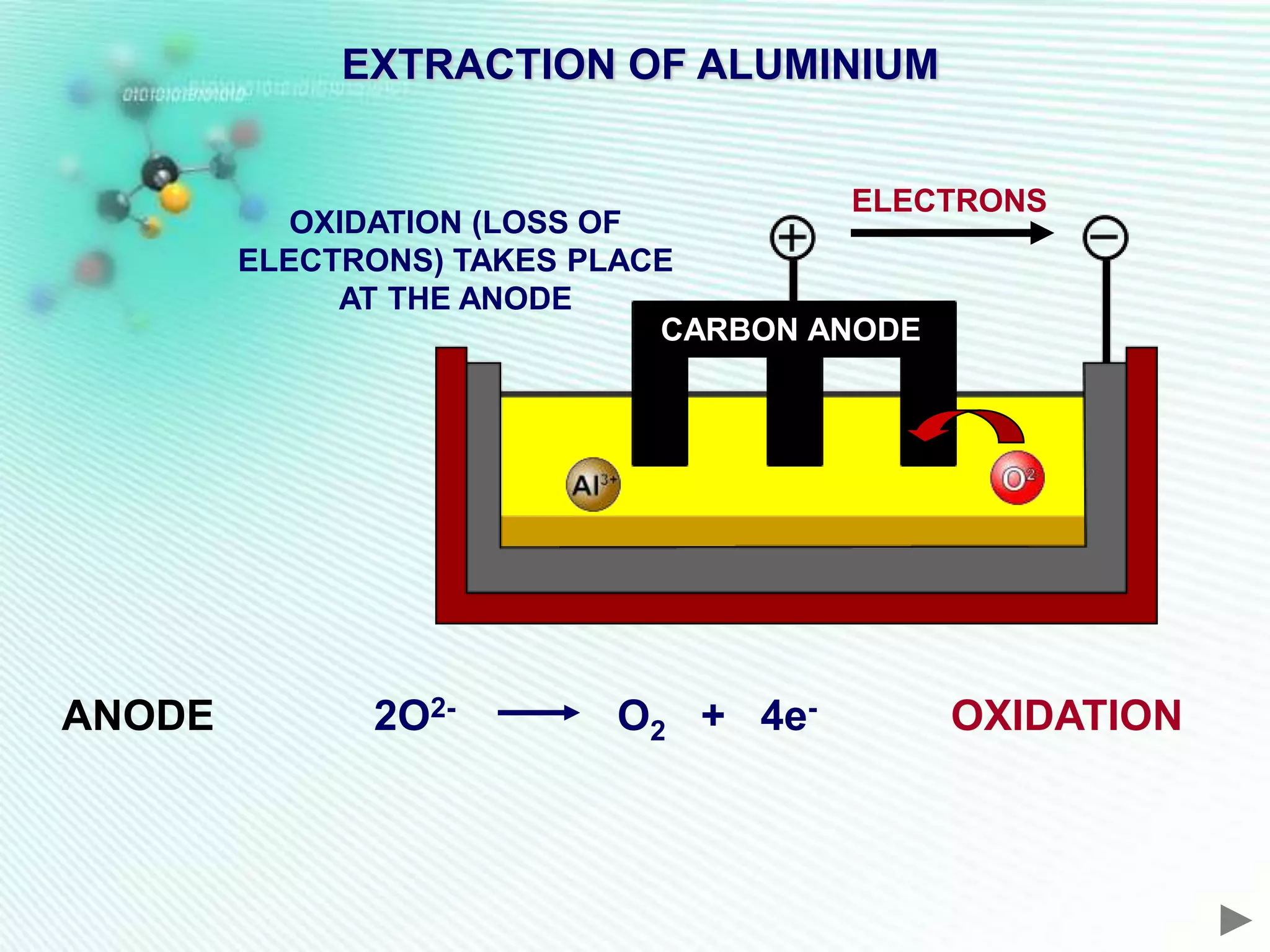
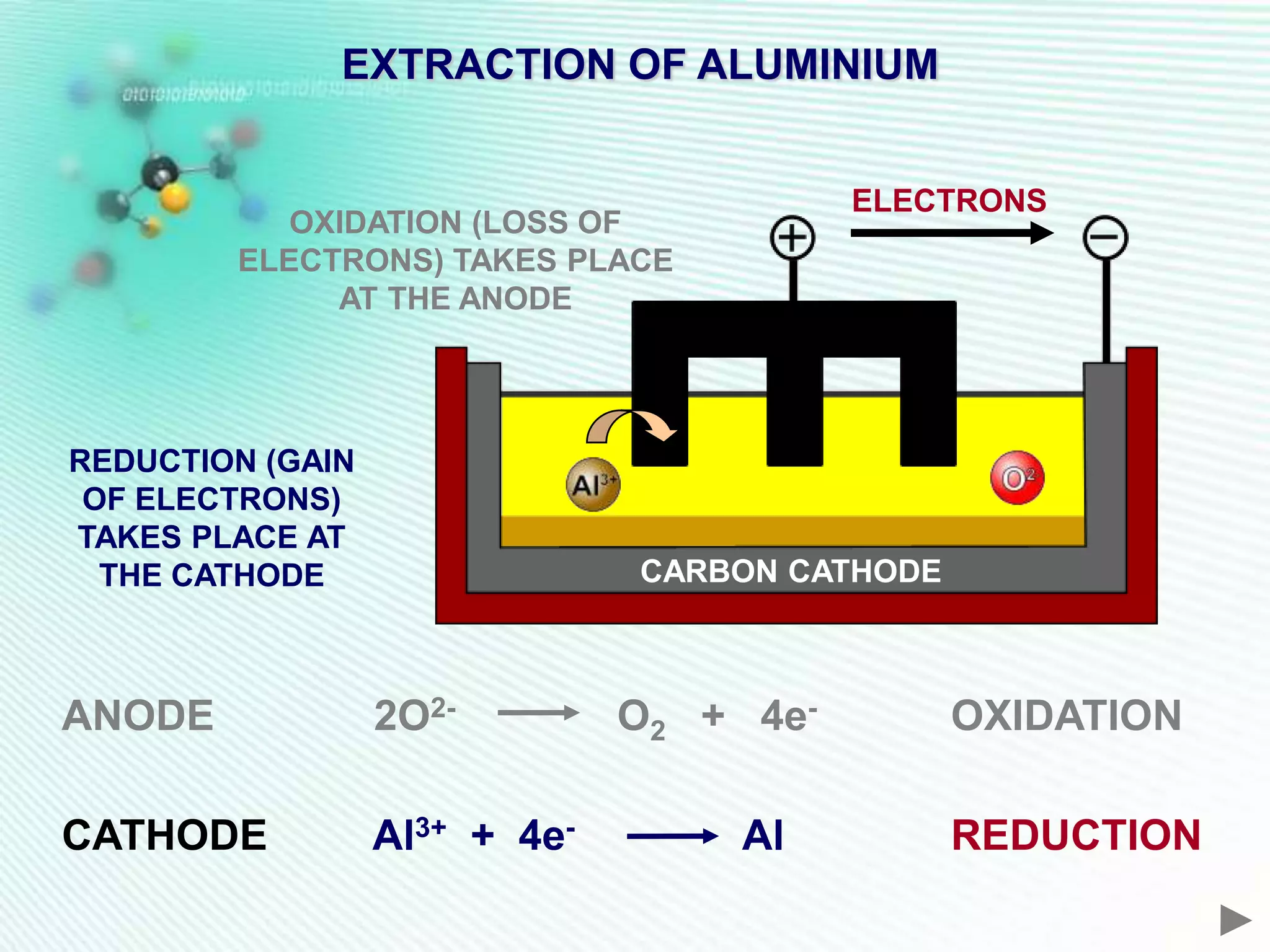
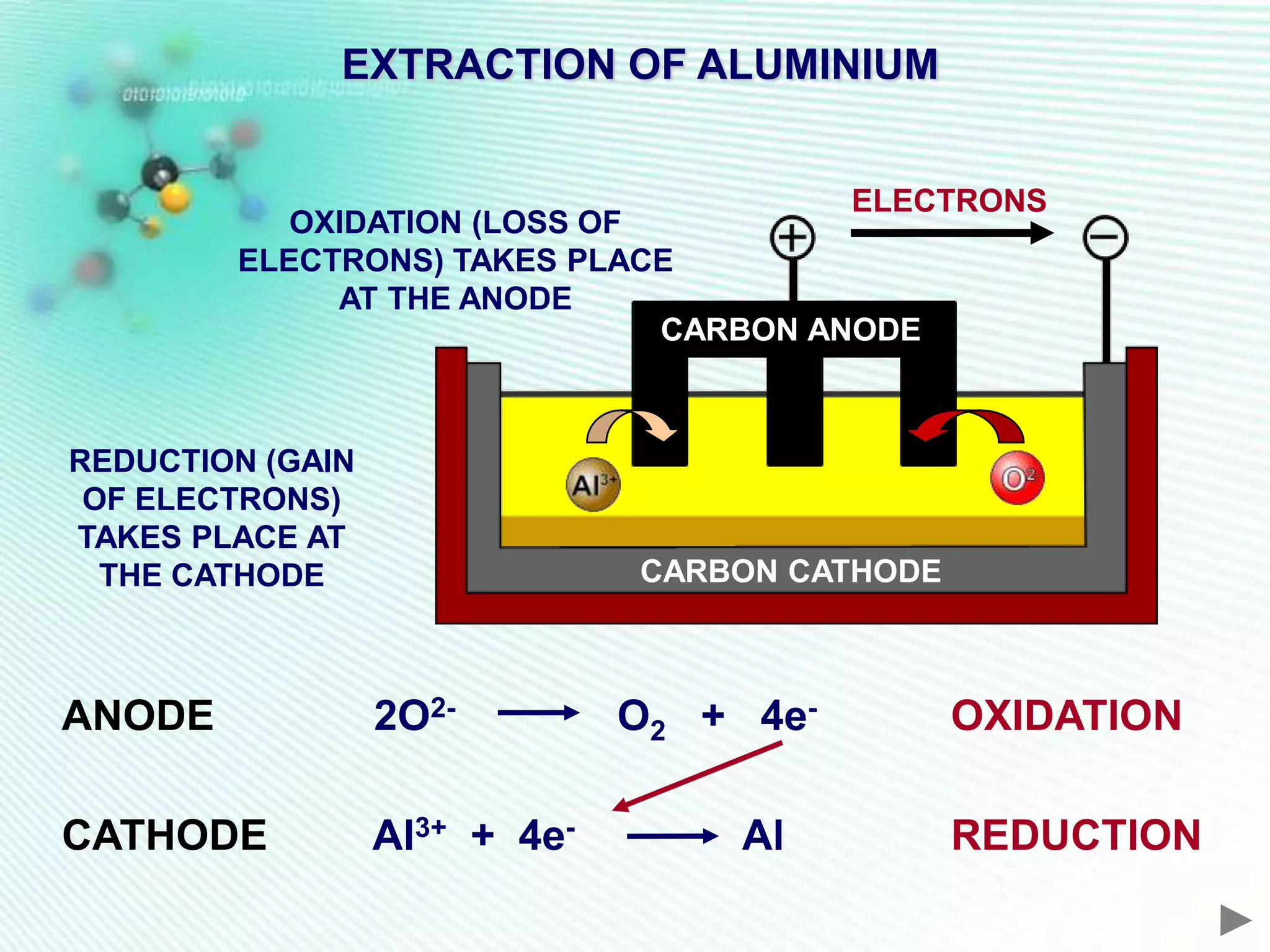
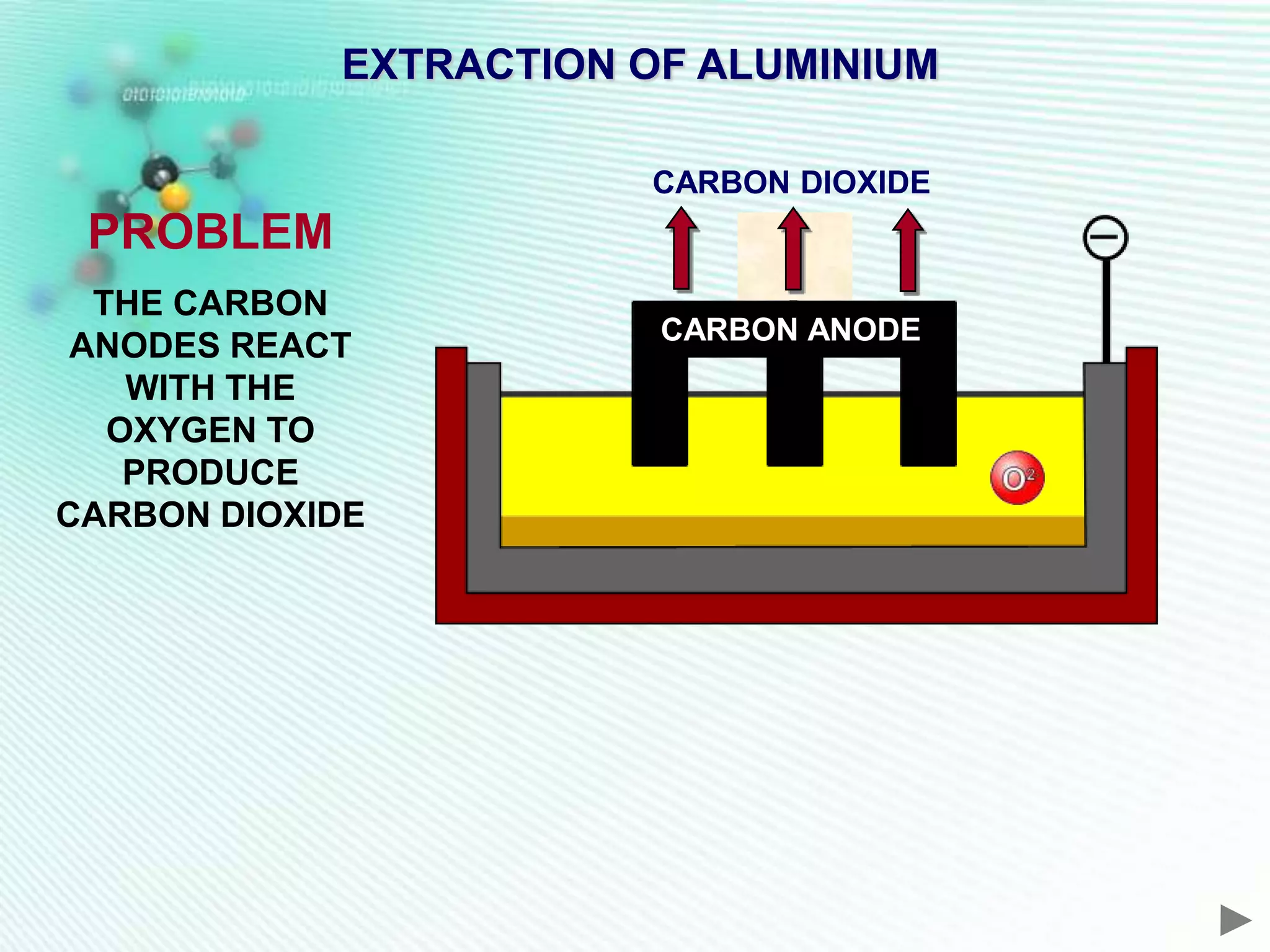
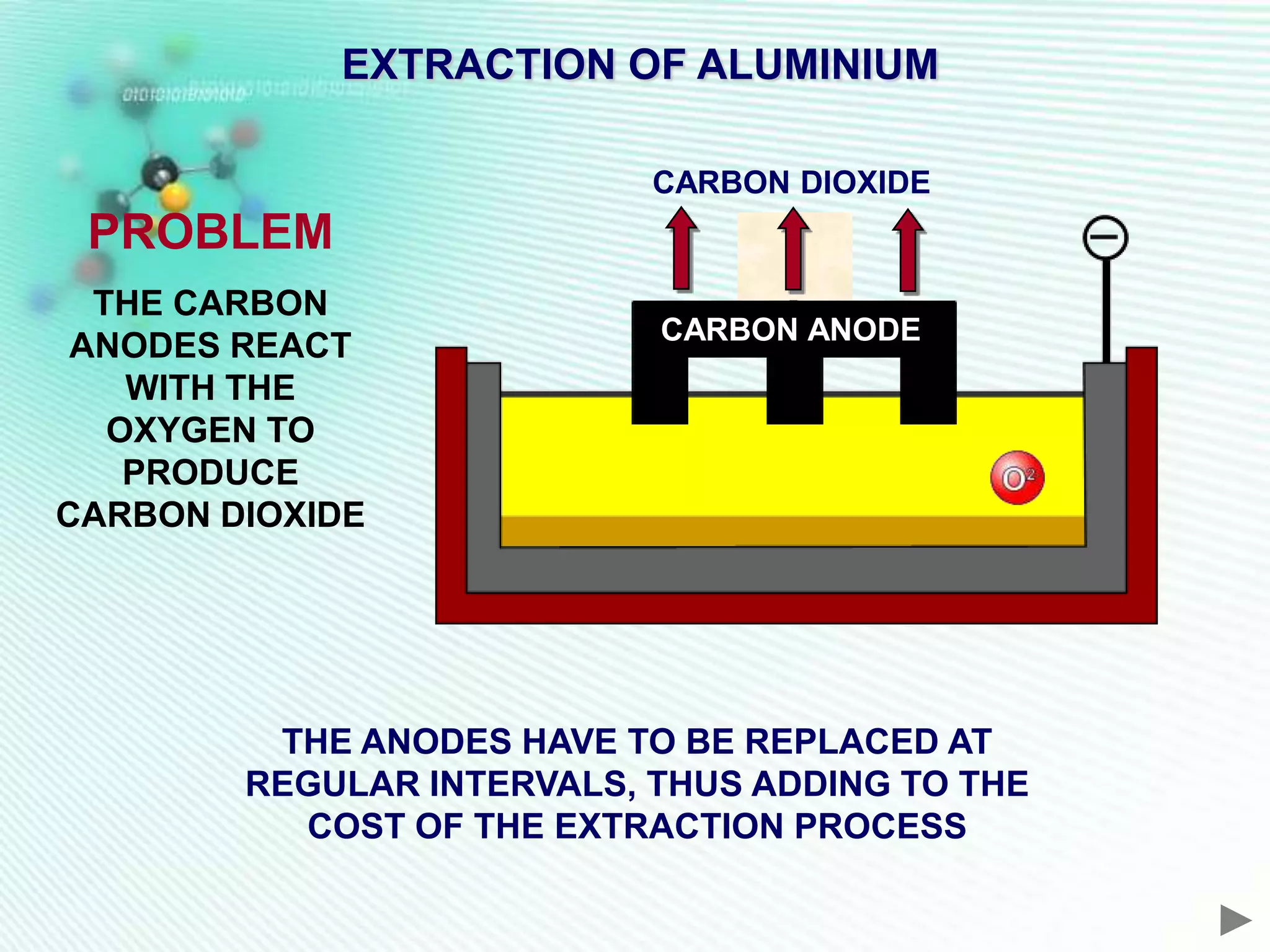
The document discusses the extraction of iron and aluminum. Iron is extracted through carbon reduction in a blast furnace, while aluminum must be extracted through electrolysis due to its high position on the reactivity series. Both processes are described in detail, including inputs, reactions, and outputs. Recycling of metals is also discussed for its environmental and economic benefits.