This document discusses the key steps in the metallurgy process:
1) Crushing and pulverization breaks down ore into powder.
2) Ore dressing or concentration removes gangue (waste rock) from powdered ore using methods like gravity separation or froth flotation.
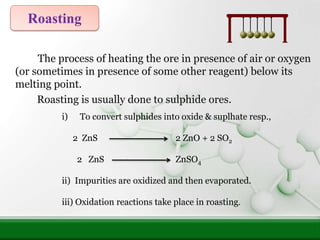
3) Calcination or roasting heats the ore to convert compounds and evaporate impurities, preparing it for smelting.
4) Smelting uses heat and chemical reducing agents to extract the pure metal from the ore by melting it and separating it from the slag waste.
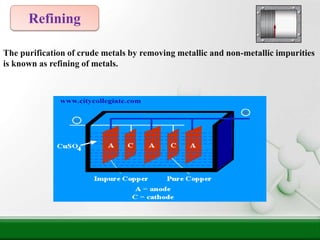
5) Refining further purifies the crude metal by removing remaining impurities.