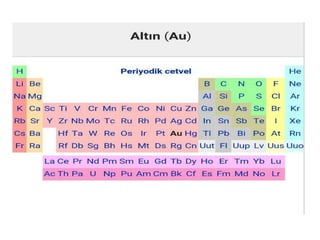
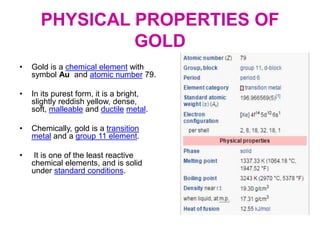
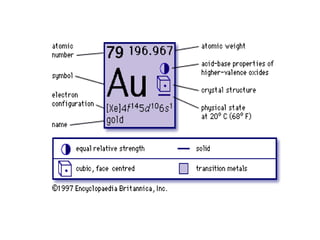
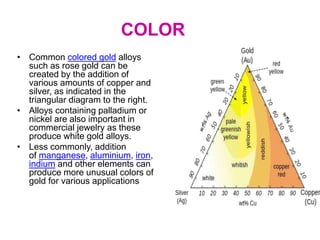
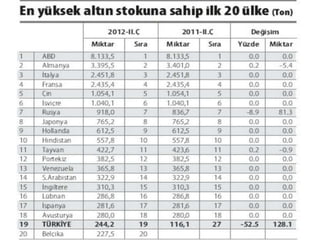
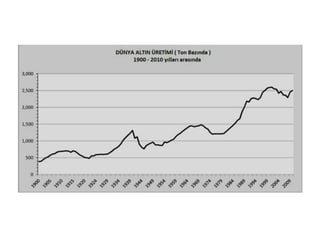
Gold is a bright yellow metal that occurs naturally in the earth. It has been used for over 5000 years for jewelry, currency and art due to its attractive color and resistance to corrosion. Gold is also used in electronics, dentistry and aerospace applications where its conductivity, malleability and resistance to corrosion are beneficial. It can also be used as a decorative ingredient in some foods and drinks.